Multiplexed proximity labelling proteomics identifies a non-canonical Bcl-2 family interactome associated with apoptotic priming
Multiplexed proximity labelling proteomics identifies a non-canonical Bcl-2 family interactome associated with apoptotic priming
Pedley, R.; Mellor, C.; King, L.; Jones, M.; Taylor-Hearn, I.; Mironov, A.; Lawless, C.; Gilmore, A. P.
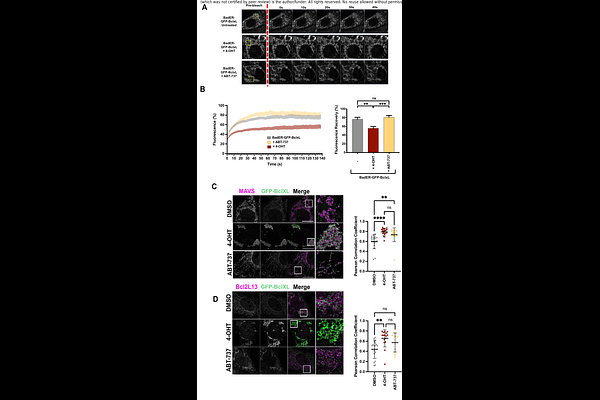
AbstractBcl-2 family proteins govern the intrinsic apoptotic pathway by regulating mitochondrial outer membrane permeabilisation (MOMP), releasing apoptogenic factors into the cytosol. How close a cell is to MOMP, termed mitochondrial priming, is determined by the interactions between different Bcl-2 proteins at the outer mitochondrial membrane (OMM). However, Bcl-2 proteins can also drive diverse processes that do not result in cell death, such as incomplete MOMP, sublethal caspase activation, pro-inflammatory signalling and regulation of cellular metabolism. To understand the wider Bcl-2 family interactome and how it might be involved in these processes, we undertook an unbiased proteomic BioID screen, using both pro- and anti-apoptotic Bcl-2 family members as bait proteins. We found that most high-confidence potential interactions in non-apoptotic cells were outside the canonical Bcl-2 family interactome. Analysis of how this interactome changed in response to either BH3-mimetics or full-length BH3-only proteins revealed dynamic changes at multiple organelles and membrane contact sites in response to altered mitochondrial priming. These findings underscore the complex and varied interactions of the Bcl-2 family proteins, expanding their scope and function beyond the frequently studies intra-family interactions.