An Adipose mTORC1 Effector Links Nutrient-induced Ribosomal Protein Translation to Lifespan in Drosophila
An Adipose mTORC1 Effector Links Nutrient-induced Ribosomal Protein Translation to Lifespan in Drosophila
Wang, J.; Cai, Z.; Gu, J.; Yang, M.; Chang, K.; Ning, X.; Wen, Y.; Yan, Y.; Lu, J.; Wang, Y.; Zhai, Z.
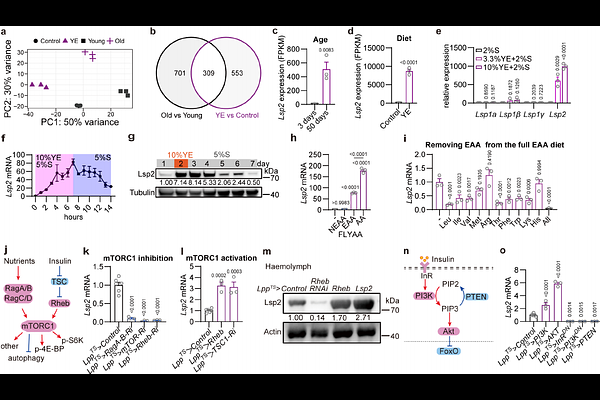
AbstractMechanistic target of rapamycin complex 1 (mTORC1) senses nutrient availability to orchestrate metabolic processes critical for physiological homeostasis and organismal ageing. While mTORC1 preferentially regulates the translation of 5\'-terminal oligopyrimidine (TOP) motif-containing mRNAs that predominantly encode ribosomal proteins (RPs) via the translational repressor 4E-BP, this mTORC1 function is resistant to rapamycin inhibition. TOP mRNAs are exceptionally abundant, thus imposing a major translational burden on cells; yet how their translation is physiologically tuned and linked to longevity remain unexplored. Here we identify Lsp2, originally known as a storage protein, as an adipose effector of mTORC1 that modulates lifespan in Drosophila. Lsp2 is induced by essential amino acids (EAAs) via mTORC1. Genetic ablation of Lsp2 to blunt organismal response to protein diets drives robust lifespan extension without compromising key life-history traits such as reproduction. Translatomic profiling reveals that loss of Lsp2 selectively reduces global TOP mRNA translation in a 4E-BP-dependent manner, thereby extending lifespan via a mechanism distinct from rapamycin inhibition that fails to reduce 4E-BP phosphorylation in vivo. Finally, Lsp2 adipokine promotes 4E-BP phosphorylation and acts systemically across tissues to shape the lifespan responses to dietary protein. Collectively, our findings establish Lsp2 as a novel translational regulator of TOP genes that mechanistically couples physiological ribosomal protein synthesis with organismal longevity.