Catalytic activity of the prepilin peptidase PilD is required for full P. aeruginosa virulence in a nematode infection model
Catalytic activity of the prepilin peptidase PilD is required for full P. aeruginosa virulence in a nematode infection model
Cabading, J. M.; Dade, C. M.; Forest, K. T.
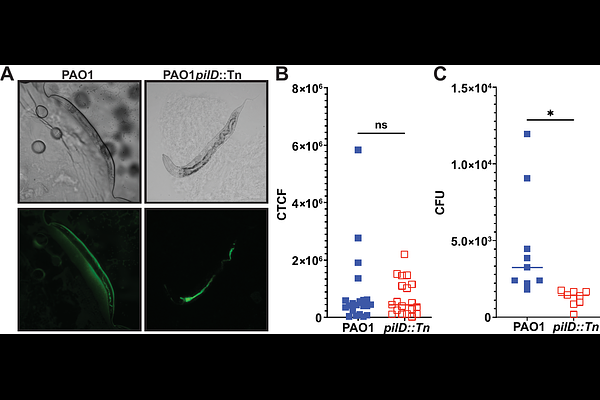
AbstractPseudomonas aeruginosa is an ESKAPE pathogen of concern because of its antibiotic resistance and ability to colonize and infect humans in myriad diverse clinical settings, from the lungs of cystic fibrosis patients to burn wounds. Antivirulence strategies have emerged as an alternative to antibiotics for treating P. aeruginosa and other pathogens. One proposed antivirulence target is the prepilin peptidase PilD because of its centrality in two virulence mechanisms: the Type IV pili and the Type II Secretion System (T2SS). Substitution of invariant aspartic acids in the putative active site of PilD led to loss of peptidase activity in an in vitro cleavage assay and abrogation of both pilus-dependent twitching motility and T2SS-dependent protease secretion. Subsequently, this study utilized a simple Caenorhabditis elegans animal infection model to investigate the in vivo magnitude of the role of PilD on P. aeruginosa virulence. In the absence of functional PilD--either through gene disruption or catalytic inactivation--P. aeruginosa exhibited delayed lethality and was reliant on other virulence mechanisms to infect and kill C. elegans. These findings highlight PilD as a valuable antivirulence target in P. aeruginosa.