Molecular Basis of EWS Interdomain Self-Association and Its Role in Condensate Formation
Molecular Basis of EWS Interdomain Self-Association and Its Role in Condensate Formation
Sohn, E. J.; Sojitra, K. A.; Rodrigues, L.; Xu, X.; Frost, B.; Mittal, J.; Libich, D. S.
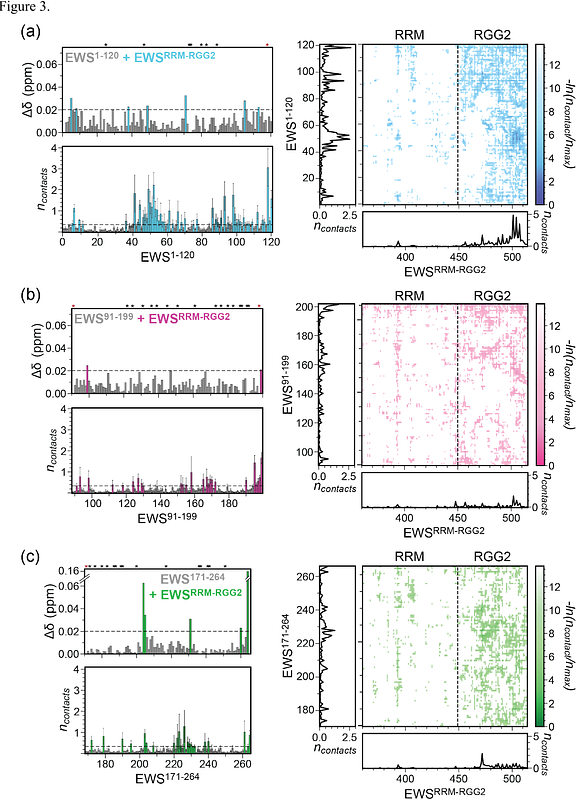
AbstractEwing sarcoma, the second most common pediatric bone and soft tissue cancer, is caused by aberrant fusion of the EWS low-complexity domain (EWSLCD) to the DNA-binding domain of the transcription factor FLI1. The resulting fusion, EWS::FLI1, directly interacts with and engages in a dynamic interplay with EWS that drives tumorigenesis and regulates the function of both proteins. While EWSLCD is known to promote self-association, the role of the RNA-binding domains (RBDs) of EWS, which include RGG repeat regions and a structured RNA-recognition motif (RRM), remains less well understood. Here, we investigate the interplay between EWSLCD and RBDs using biomolecular condensation assays, microscopy, NMR spectroscopy, and molecular simulations. Our studies reveal that RBDs differentially influence EWSLCD condensate formation and suggest that electrostatics and polypeptide-chain length likely contribute to this interaction. NMR spectroscopy and molecular dynamics simulations further demonstrate that EWSLCD and the central RNA-binding region, comprising the RRM and RGG2 domains, engage in transient, non-specific interactions that are broadly distributed across both regions and involve diverse residue types. Specifically, tyrosine, polar residues, and proline within EWSLCD preferentially interact with arginine, glycine, and proline residues in the RBD. Atomistic simulations of EWS confirm that the full-length protein exhibits a similar interaction profile with conserved chemical specificity, supporting a model in which a network of weak, distributed interdomain contacts underlies EWS self-association. Together, these findings provide molecular insight into the mechanisms of EWS condensate formation and lay the groundwork for understanding how interdomain interactions regulate EWS and EWS::FLI1 function.