Structural modeling reveals the allosteric switch controlling the chitin utilization program of Vibrio cholerae
Structural modeling reveals the allosteric switch controlling the chitin utilization program of Vibrio cholerae
Anderson, H. S.; Dalia, A. B.
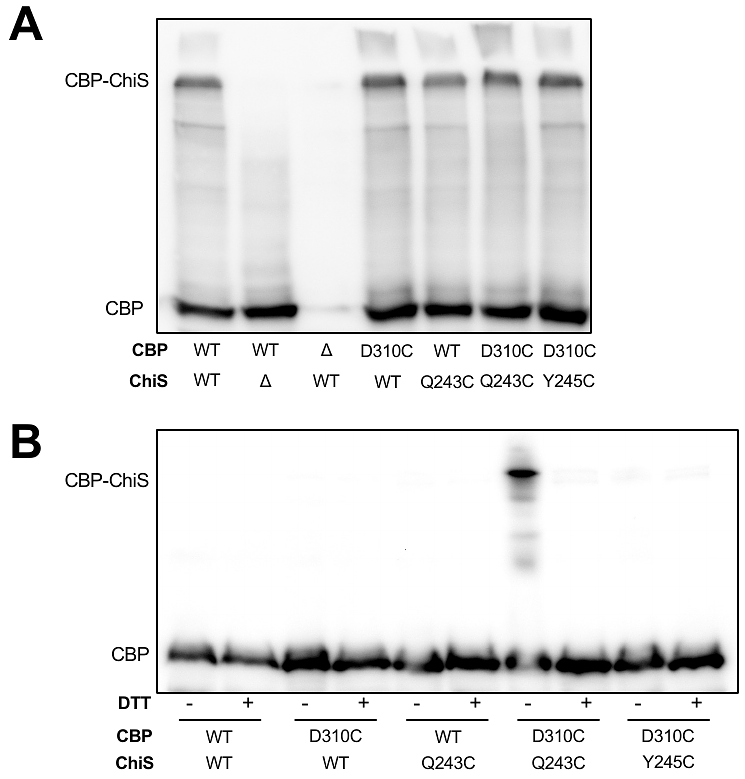
AbstractSignal transduction by histidine kinases (HKs) is nearly ubiquitous in bacterial species. HKs can either sense ligands directly or indirectly via a cognate substrate binding protein (SBP). The molecular basis for SBP-dependent signal reception, however, remains poorly understood in most cases. CBP and ChiS are the SBP-HK pair that activate the chitin utilization program of Vibrio cholerae. Here, we elucidate the molecular basis for allosteric regulation of CBP-ChiS by generating structural models of this complex in the unliganded and liganded states, which we support with extensive genetic, biochemical, and cell biological analysis. Our results reveal that ligand-binding induces a large conformational interface switch that is distinct from previously described SBP-HKs. Structural modeling suggests that similar interface switches may also regulate other uncharacterized SBP-HKs. Together, these results extend our understanding of signal transduction in bacterial species and highlight a new approach for uncovering the molecular basis of allostery in protein complexes.