Increased EThcD efficiency on the hybrid Orbitrap Excedion Pro Mass Analyzer extends the depth in identification and sequence coverage of HLA class I immunopeptidomes
Increased EThcD efficiency on the hybrid Orbitrap Excedion Pro Mass Analyzer extends the depth in identification and sequence coverage of HLA class I immunopeptidomes
Kessler, A. L.; Fort, K.; Resemann, H.; Krueger, P.; Wang, C.; Koch, H.; Hauschild, J.-P.; Marino, F.; Heck, A. J. R.
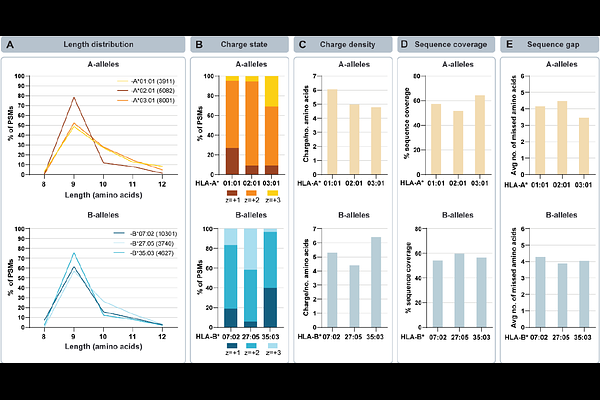
AbstractGaining a complete and unbiased understanding of the non-tryptic peptide repertoire presented by HLA-I complexes by LC-MS/MS is indispensable for therapy design for cancer, autoimmunity and infectious diseases. A serious concern in HLA peptide analysis is that the routinely used, collision-based fragmentation methods (CID/HCD) do not always render sufficiently informative MS2 spectra, whereby gaps in the fragmentation sequence coverage prevent unambiguous assignments. EThcD can be utilized to generate complementary ion series, i.e. b/y ions and c/z ions, resulting in richer, more informative MS2 spectra, thereby filling in the gaps. Here, we present data generated on a novel hybrid Orbitrap mass spectrometer, facilitating fast and efficient hybrid fragmentation due to the implementation of EThcD in the ion routing multipole. We hypothesized that this would enable more comprehensive and less error-prone analysis of immunopeptidomes at minimal costs in duty-cycle. First, we optimized ETD/EThcD methods using an elastase-digested cell lysate, as this contains peptides of similar length and charge distributions to immunopeptides. Next, we compared HCD and EThcD on immunopeptidomes originating from three cell lines with distinct HLA-I complexes that present peptides with varying physicochemical properties. We demonstrate that the new instrument not only enables efficient and fast ETD reactions, but when combined with collision-based supplemental activation, i.e. EThcD, also consistently increases the sequence coverage and identification of peptide sequences, otherwise missed by using solely HCD. We reveal several of the biochemical properties that make HLA peptides preferably identifiable by EThcD, with internal Arg residues being one of the most dominant determinants. Finally, we demonstrate the power of EThcD for the identification and localization of HLA peptides harboring post-translational modifications, focusing here on HLA Arg mono-/di-methylation. We foresee that this new instrument with efficient EThcD capabilities enhances not only immunopeptidomics analysis, but also analysis of peptides harboring post-translational modifications and de novo sequencing.