Impact of maternal compensation on developmental phenotypes in a zebrafish model of severe congenital muscular dystrophy
Impact of maternal compensation on developmental phenotypes in a zebrafish model of severe congenital muscular dystrophy
Flannery, K. P.; Mowla, S.; Battula, N.; Clark, L. R.; Liu, D.; Oliveira, C. D.; Venkatesan, C.; Simhon, L. M.; Karas, B. F.; Terez, K. R.; Burbano Lombana, D.; Manzini, M. C.
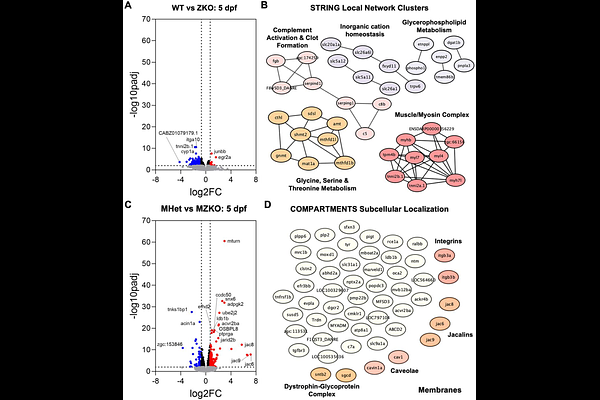
AbstractGenetic compensation is a common phenomenon in zebrafish in response to genetic alterations. As such, differences between morphant and mutant zebrafish models of human diseases have led to significant difficulties in phenotypic interpretation and translatability. One form of compensation is the maternal deposit of mRNAs and proteins into the oocyte that supports developmental processes before zygotic genome activation. In this study, we generated a zebrafish model of severe congenital muscular dystrophy by targeting protein O-mannose N-Acetylglucosaminyltransferase 2 (pomgnt2), a maternally provided gene that maintains cell-extracellular matrix interactions through glycosylation. Zygotic knockouts (ZKOs) retain protein function in the first week post-fertilization and survive to adulthood, though they develop muscle disease later in life. In contrast, maternal-zygotic KOs (MZKOs) generated from ZKO females develop early-onset muscle disease, reduced motor function, neuronal axon guidance deficits, and retinal synapse disruptions, recapitulating features of the human presentation. While assessing transcriptional changes linked to disease progression, the availability of embryos obtained from different breeding strategies also allowed for direct comparison of ZKOs and MZKOs to define the impact of having a KO mother. We found that offspring from a ZKO mother, independently of genotype, show distinct expression patterns from animals obtained from heterozygous breeding. Some of these changes reflect an increased metabolic requirement, possibly stemming from maternal metabolic disruption. These findings will not only be applicable to other CMD models targeting maternally provided genes but also provide new insight into modeling disease using maternal-zygotic mutants.