Deregulated Myt3 translation predisposes islet β-cells to dysfunction under obesity-induced metabolic stress
Deregulated Myt3 translation predisposes islet β-cells to dysfunction under obesity-induced metabolic stress
Hu, R.; Wang, Y.; Yagan, M.; Xu, Y.; Simmons, A.; Lau, K.; Liu, Q.; Gu, G.
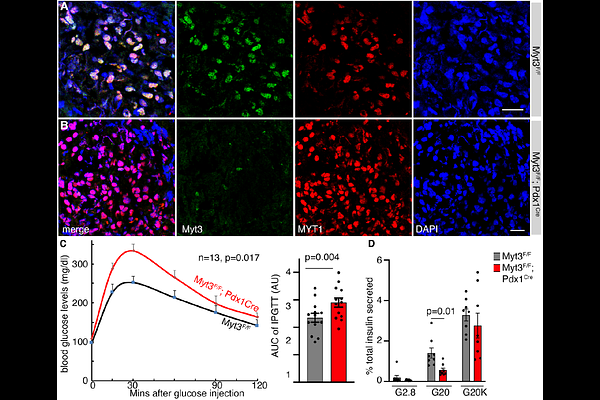
AbstractIn response to obesity-related metabolic stress, islet {beta}-cells adapt (or compensate) by increasing their secretory function and mass. Yet, for unknown reasons, this compensation is reversed in some individuals at some point to induce {beta}-cell failure and overt type 2 diabetes (T2D). We have previously shown that transcription factor Myt3 (St18) and its paralogs, Myt1 and Myt2, prevent {beta}-cell failure. Myt3 was induced at post-transcriptional levels by obesity-related stress in both mouse and human {beta} cells and its downregulation accompanied {beta}-cell dysfunction during T2D development. Single-nucleotide polymorphisms in MYT3 were associated with an increased risk of developing human diabetes. We now demonstrate that Myt3 translation is regulated by an upstream open-reading frame that overlaps with the main Myt3 open-reading frame in mice. Disrupting this overlap enhances Myt3 translation in mouse {beta} cells without metabolic stress but decreases it under high-fat-diet challenges. Consequently, this deregulation results in {beta}-cell dysfunction and glucose intolerance in mice, accompanied by compromised expression of several {beta}-cell function genes. These findings suggest that stress-induced Myt3 translation is part of the compensation mechanism that prevents {beta}-cell failure.