A Set of Novel Far-Red Fluorescent Proteins for Temporal Domain Multiplexing and Super-Resolution Imaging
A Set of Novel Far-Red Fluorescent Proteins for Temporal Domain Multiplexing and Super-Resolution Imaging
Olumayowa, F.; Rong, Z.; Wang, R.; Papadaki, S.; Wang, X.; Cao, J.; Subach, F. V.; Koster, R. W.; Namikawa, K.; Piatkevich, K. D.
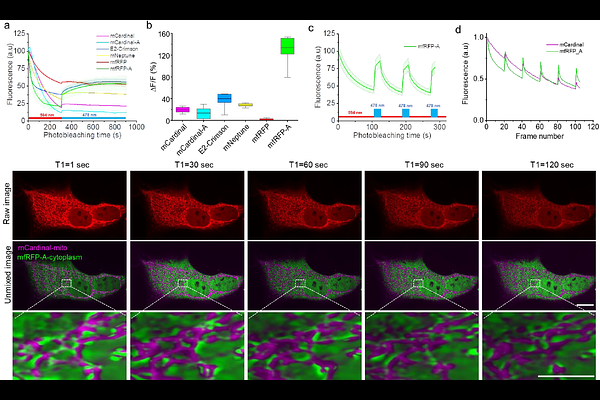
AbstractThe diverse spectral and photochemical properties of fluorescent proteins enable a variety of imaging applications in cell biology, ranging from cellular and organelle labeling to super-resolution microscopy and multiplexed live cell imaging. Here, we report a set of novel far-red fluorescent proteins, named mfRFP, mfRFP-A, and mCardinal-A, which are characterized by similar fluorescence spectra with excitation/emission at ~600/660 nm while exhibiting distinct photobleaching rates. Differences in photostability allowed us to perform per-pixel unmixing of the three far-red FPs imaged simultaneously by employing a recently introduced temporal domain multiplexing approach. We demonstrated the application of the temporal domain multiplexing approach with different combinations of far-red fluorescent proteins possessing nearly identical emission spectra by acquiring BrainBow-like images of cellular populations and distinguishing subcellular structures in mammalian cells using a single imaging channel without applying any hardware modifications to the conventional microscope. Unlike previous temporal domain multiplying approaches employing photophysical properties of fluorescent proteins, the current approach is a wide range of microscopy modalities, including 3D imaging with a spinning disk and point scanning confocal microscopy. The most photostable fluorescent protein in the set, mfRFP, was further benchmarked against spectrally similar FPs and applied for super-resolution imaging of structural proteins in mammalian cells and for neuroimaging of model organisms, including mice, zebrafish, and C. elegans.