RNase H1 counteracts DNA damage and ameliorates SMN-dependent phenotypes in a Drosophila model of Spinal Muscular Atrophy
RNase H1 counteracts DNA damage and ameliorates SMN-dependent phenotypes in a Drosophila model of Spinal Muscular Atrophy
Scatolini, L.; Graziadio, L.; Guerrini, D.; Maccallini, P.; Marino, C.; di Vito, R.; Hassan, A.; Grimaldi, M.; Fidaleo, M.; Dehghannasiri, R.; Proietti, G.; Piergentili, R.; Salvati, E.; Cacchione, S.; De Pitta, C.; Doksani, Y.; D'Ursi, A. M.; Wakefield, J.; Chen, L.; Gatti, M.; Colantoni, A.; Usiello, A.; Raffa, G. D.
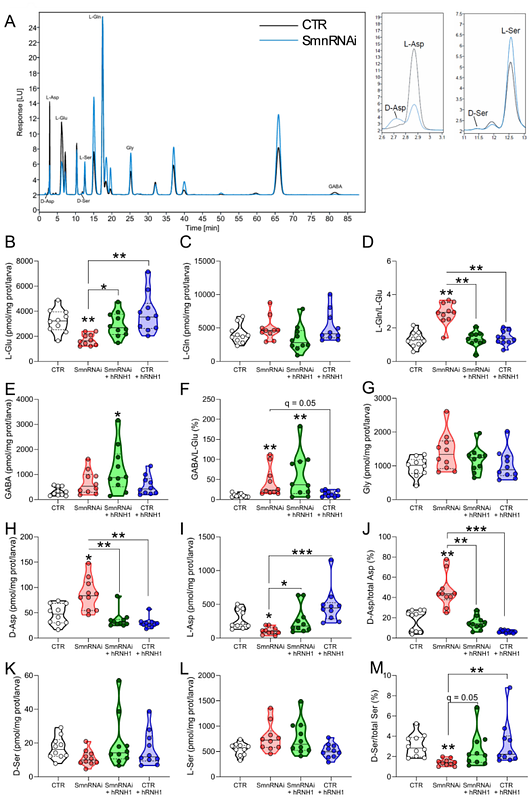
AbstractSpinal Muscular Atrophy (SMA) is caused by a deficiency of the Survival Motor Neuron (SMN) protein. Mutations in SMN disrupt mRNA splicing and translation, leading to maladaptive changes in transcriptomes, proteomes, neuroinflammation, and metabolism, which drive motor neuron degeneration in SMA patients. Using a Drosophila SMA model, we found that systemic depletion of Smn leads to accumulation of RNA:DNA hybrids (Rloops), increased DNA damage, dysregulation of amino acids and sugar metabolism and activation of the innate immune response, recapitulating key pathological features reported in mammalian models and severe SMA patients. Persistent DNA damage in Smn-deficient flies alters cell proliferation rates in larval brains and induces extensive cell death in the developing eye. Importantly here, we show that stimulating the resolution of RNA:DNA hybrids with transgenic human RNAse H1 prevents the accumulation of DNA damage and attenuates the transcriptome and amino acid alterations induced by Smn depletion, mitigating the Smn-dependent cellular and developmental abnormalities, in Smn-deficient flies. Our data suggest that depletion of Smn causes an accumulation of aberrant transcripts and chronic DNA damage, which along with the altered metabolomic profiles associated with Smn deficiency trigger systemic inflammatory responses, ultimately affecting neuronal function and survival.