Arp2/3 complex contributes to the actin-dependent uptake of Aspergillus terreus conidia by alveolar epithelial cells
Arp2/3 complex contributes to the actin-dependent uptake of Aspergillus terreus conidia by alveolar epithelial cells
Mach, N.; Polleux, J.; Heinrich, L.; Lechner, L.; Levytska, I.; Lass-Floerl, C.; Perkhofer, S.
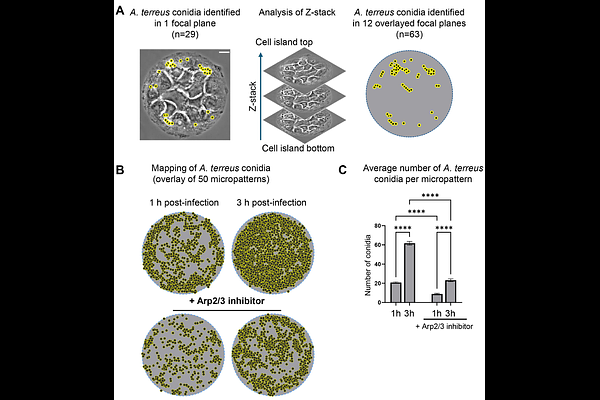
AbstractAspergillus terreus is an opportunistic fungal pathogen associated with high mortality rates and intrinsic resistance to amphotericin B. Its ability to persist within host tissues without inducing strong immune responses was suggested to contribute to poor clinical outcomes. The cellular mechanisms underlying A. terreus interactions with host cells remain largely unexplored. In this study, we have used a micropattern-based infection model to investigate the early interactions between A. terreus conidia and alveolar epithelial cells, focusing on the role of Arp2/3-dependent actin remodeling. This system allows quantitative analysis of conidia-cell interactions under defined spatial conditions. We show that A. terreus conidia rapidly bind to micropatterned A549 cell islands, with conidial numbers increasing over time. Conidia were found in actin- and Lamp1-positive vesicles already after one hour of infection. Inhibition of the Arp2/3 complex significantly impaired conidial binding and disrupted the formation of actin-positive vesicles, confirming the essential role of Arp2/3-mediated actin remodeling in early stages of conidial uptake. A subset of internalized conidia was localized to Lamp1-positive phagolysosomes and accumulated over time. Interestingly, we have identified a small but consistent population of phagolysosomes decorated with actin patches, potentially resembling actin flashes. These structures were entirely abolished upon Arp2/3 inhibition, indicating active cytoskeletal remodeling at the phagolysosomal interface. Our findings provide the first mechanistic insights into A. terreus internalization by alveolar epithelial cells and establish Arp2/3-mediated actin dynamics as a key process in early host-pathogen interactions. This cellular pathway may contribute to intracellular persistence and help understand the delayed onset of A. terreus infections.