Translatome profiling reveals opposing alterations in inhibitory and excitatory neurons of Fragile X mice and identifies EPAC2 as a therapeutic target.
Translatome profiling reveals opposing alterations in inhibitory and excitatory neurons of Fragile X mice and identifies EPAC2 as a therapeutic target.
Suresh, A.; Kourdougli, N.; Buth, J. E.; Sanchez-Leon, C. A.; Wall, L. T.; Tran, A. T.; Miranda-Rottmann, S.; Araya, R.; Gandal, M. J.; Portera-Cailliau, C.
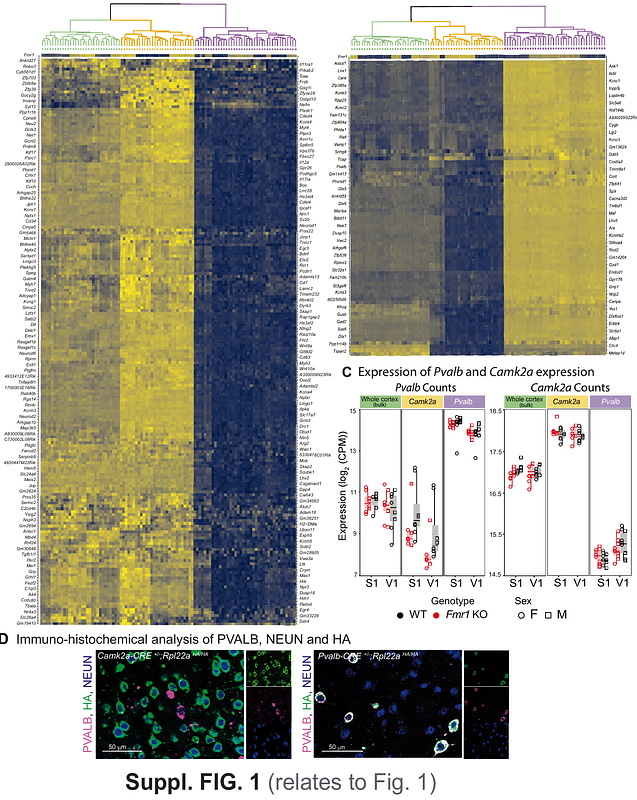
AbstractSymptoms of Fragile X Syndrome (FXS), the leading monogenic cause of intellectual disability and autism, are thought to arise from an excitation/inhibition (E/I) imbalance. Here, we leverage cell type specific mRNA sequencing to profile molecular alterations of cortical excitatory and inhibitory neurons in Fmr1 knockout (KO) mice, integrating transcriptomic results with circuit and behavioral readouts to prioritize novel therapeutic targets. Differentially expressed genes (DEG) were largely upregulated in Camk2a expressing excitatory neurons but downregulated in Pvalb-expressing inhibitory neurons, and the underlying signaling pathways were often altered in opposite directions. Among the 184 DEGs that were concordantly dysregulated across both cell types, only Rapgef4 (Epac2) was also an FMRP target, an ASD risk gene and brain enriched. EPAC2 has been implicated in synaptic maturation and plasticity. Systemic administration of an EPAC2 antagonist restored cortical circuit function in Fmr1 KO mice and ameliorated sensory behavioral phenotypes. EPAC2 is a potential target for therapy in FXS.