Gestational Chronic Intermittent Hypoxia Triggers Maternal Inflammation and Disrupts Placental Stress Responses
Gestational Chronic Intermittent Hypoxia Triggers Maternal Inflammation and Disrupts Placental Stress Responses
Gardner, J. J.; Oliveira da Silva, R. d. N.; Bradshaw, J. L.; Mabry, S.; Wilson, E. N.; Hula, N.; Tucker, S. M.; Gorham, I. K.; Escalera, D.; Lopez, L.; Phillips, N. R.; Cunningham, R. L.; Goulopoulou, S.
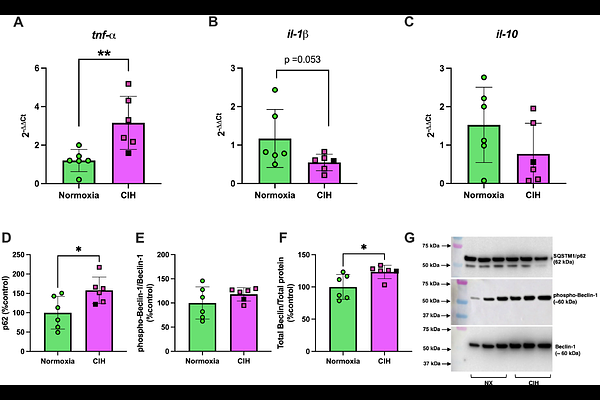
AbstractGestational hypoxia is associated with placental cellular responses, including oxidative stress and inflammation. Circulating cell-free mitochondrial DNA (ccf-mtDNA) is a marker of cell stress, that can be transported within extracellular vehicles (EVs), eliciting proinflammatory responses. We hypothesized that systemic exposure to chronic intermittent hypoxia (CIH) during late pregnancy would increase maternal inflammation, alter circulating EV characteristics, and disrupt placental stress responses. Pregnant rats were exposed to CIH (n=8) or normoxia (n=9) during gestational days 15-20 (term 22-23 days). On GD20, ccf-mtDNA and EV-associated mtDNA (EV-mtDNA) were quantified with qRT-qPCR, while maternal circulating cytokines were quantified using a MILLIPLEX(R) cytokine array. Systemic oxidative stress was measured by plasma advanced oxidation protein products (AOPP). Placental stress responses were evaluated by examining the balance between proinflammatory and antioxidant gene expression and the activation of proteins involved in apoptotic and autophagic processes. CIH exposure increased placental weights (p=0.015) and reduced placental efficiency (p=0.0006) without affecting fetal biometrics (p>0.05). Absolute ccf-mtDNA and EV-mtDNA content were unchanged (p>0.05), but EV concentrations were reduced (p=0.011) in response to CIH, suggesting an increase in EV-mtDNA per EV. Maternal interleukin-18 (IL-18) concentrations increased in the CIH group (p=0.047). Placental mRNA expression of catalase (p=0.048) and sod2 (p=0.038) were upregulated, while autophagy-related proteins Beclin-1 (p=0.006) and p62 (p=0.023) were also increased in response to CIH, with no changes in LC3A/B expression (p>0.05). Gestational CIH disrupts maternal EV and inflammatory profiles, reduces placental efficiency, and modulates placental antioxidant and autophagic mechanisms, without impairing fetal growth in rats.