Modulation of Filopodial Myosin Function
Modulation of Filopodial Myosin Function
Crawford, A.; Schwartz, J. K.; Hall, E.; Houdusse, A.; Titus, M. A.
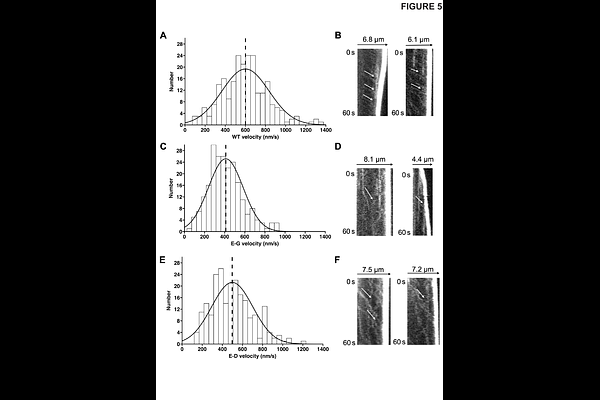
AbstractMyTH-FERM (MF) myosins are essential for filopodia formation in both Metazoa and Amoebozoa (Dictyostelium DdMyo7; mammalian Myo10) but their roles in filopodia formation are not fully understood. Taking advantage of a mutation in the highly conserved actin binding interface of another MF myosin, the jordan (jd) mutation of Myo15A that reduces its function, the impact of altering the myosin-F-actin interaction on filopodia formation was investigated. The jd mutation, a D to G substitution (Gong et al, 2022, Sci Adv), was introduced into DdMyo7 or Myo10 and filopodia formation assessed by quantitative analysis of number, length and myosin tip enrichment. The mutation significantly decreased filopodia initiation and tip intensity of both DdMyo7 and Myo10. This is consistent with impairment of myosin-based reorganization of cortical actin at the cortex, with myosins in the initiation complex remaining at the tip as the filopodium extends. The DdMyo7 jd mutation did not affect filopodia length. In the case of Myo10, while the jd mutation reduced Myo10 velocity of intrafilopodial motility by 40%, resulting in reduced levels of myosin at the filopodia tip, the jd mutant did not consistently affect the length of filopodia in different cell lines. This indicates that the major role of Myo10 may not be to promote filopodia elongation by reducing membrane tension, as has been proposed. Rather, the phenotype of the DdMyo7 and Myo10 jd mutants indicates that the major role of evolutionarily diverse filopodial myosins is to re-organize actin filaments at the membrane-cortex interface during the critical initiation step.