A Conserved Mycobacterial Nucleomodulin Hijacks the Host COMPASS Complex to Reprogram Pro-Inflammatory Transcription and Promote Intracellular Survival
A Conserved Mycobacterial Nucleomodulin Hijacks the Host COMPASS Complex to Reprogram Pro-Inflammatory Transcription and Promote Intracellular Survival
Chen, L.; Duan, B.; Chen, P.; Jiang, Q.; Wang, Y.; Lu, L.; Chen, Y.; Hu, C.; Zhang, L.; Guo, A.
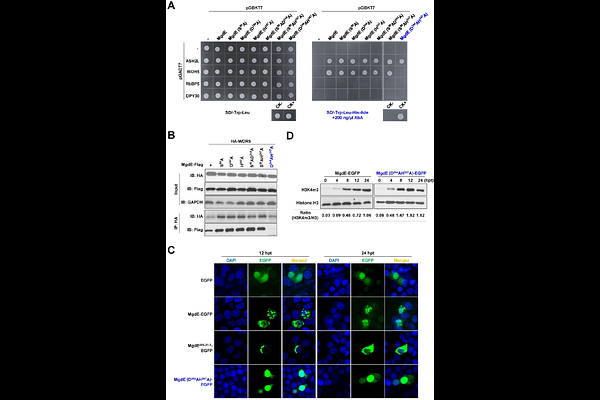
AbstractNucleomodulins are a class of effector proteins secreted by bacterial pathogens that translocate into the host cell nucleus to modulate nuclear processes. However, their target proteins and underlying molecular mechanisms remain poorly understood in mycobacteria. Herein, we identified a conserved hypothetical protein Rv1075c, designated MgdE, as a nucleomodulin that enhances mycobacterial intracellular survival. MgdE undergoes nuclear translocation via two nuclear localization signals, KRIR108-111 and RLRRPR300-305, and interacts with ASH2L and WDR5, two subunits of the host histone methyltransferase COMPASS complex. This interaction suppresses histone H3 lysine 4(H3K4) methylation-mediated transcription of pro-inflammatory genes, including il6 and il1{beta}, thereby promoting mycobacterial survival in both macrophages and mice. Our study provides the first experimental evidence that a bacterial nucleomodulin facilitates intracellular survival by directly targeting the host COMPASS complex. These findings advance our understanding of mycobacterial pathogenesis by revealing a novel mechanism that contributes to its intracellular survival strategy.