Mechanical loading reveals cell type-specific responses and role of PHGDH in endothelial cell growth
Mechanical loading reveals cell type-specific responses and role of PHGDH in endothelial cell growth
Mäntyselkä, S.; Niemi, E.; Ylä-Outinen, L.; Kolari, K.; Uusitalo-Kylmälä, L.; Ortega-Alonso, A.; Liimatainen, R.-M.; Fachada, V.; Permi, P.; Kalenius, E.; Hulmi, J.; Kivelä, R.
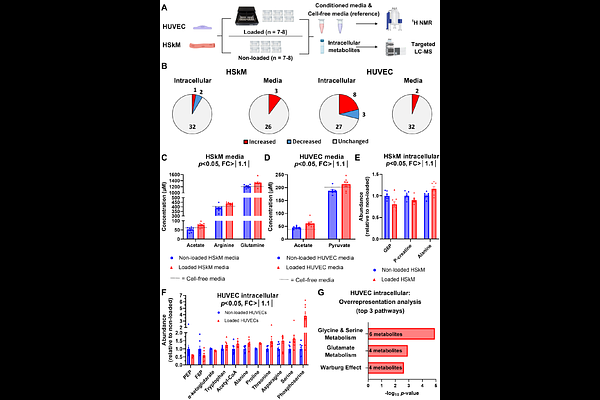
AbstractSkeletal muscle and blood vessels are constantly subjected to mechanical forces, especially during exercise. We subjected human endothelial cells (ECs) and muscle cells (MCs) to cyclic mechanical stretch and investigated acute transcriptomic and metabolomic changes. Mechanical loading induced several responses typically seen after exercise in vivo. Both cell types released acetate in response to stretching, but surprisingly, many of the other changes were in opposite directions. For instance, electron transport chain genes were downregulated in ECs and upregulated in muscle cells. Additionally, ECs shifted their transcriptomic profile to a growth-oriented direction and 13C-(U)-glucose tracing experiments revealed increased serine synthesis in ECs. Mechanistic experiments related to the serine synthesis pathway enzyme phosphoglycerate dehydrogenase (PHGDH) revealed its importance in EC anabolism. Our data suggest that mechanical loading induces cell type-specific responses in ECs and MCs, and several of these effects recapitulate exercise effects in vivo. In ECs, mechanical loading activates the serine synthesis pathway, and the serine-synthesizing enzyme PHGDH was highlighted as essential in regulating EC growth, a process indispensable in angiogenesis.