Global mapping of thioredoxin interacting proteins in Neurospora crassa
Global mapping of thioredoxin interacting proteins in Neurospora crassa
Bidondo, L.; Drula, E.; GAILLARD, j.-c.; Armengaud, J.; Berrin, J.-G.; Rosso, M.-N.; Tarrago, L.
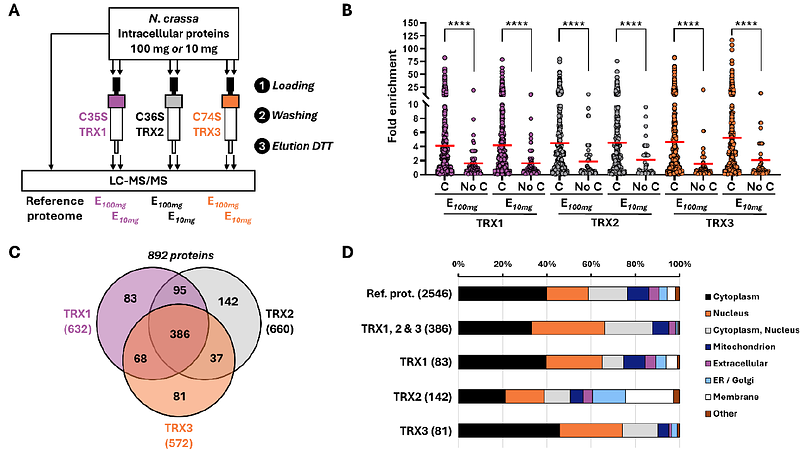
AbstractThioredoxins (Trx) are essential thiol-oxidoreductases that regulate redox homeostasis by reducing oxidized cysteines in a wide range of target proteins. However, the Trx system and other redox regulation mechanisms remain poorly characterized in saprotrophic filamentous fungi. Here, we identified the components of the Neurospora crassa Trx system and uncovered potential redox-regulated proteins using Trx affinity chromatography. Genome search identified three Trx and a single thioredoxin reductase that we named TRX1, TRX2 TRX3 and TRR. Notably, TRX1 carries a C-terminal disordered extension of unknown function, conserved in two ascomycete taxa (Leotiomycetes and Sordariomycetes). Using recombinant cysteine-to-serine mutants of each Trx, we performed affinity chromatography and identified 1,998 proteins - approximately 19% of the N. crassa proteome. To rank the putative Trx targets, we applied a fold enrichment metric, comparing protein abundance before and after affinity chromatography. The average fold enrichment was four, with values reaching up to 117 for the most enriched protein, a DEAD/DEAH box helicase. Among the top-enriched proteins, we identified homologs of known human and plant Trx targets, like peroxiredoxins, as well as 93 transcription factors and 38 kinases. Additional potential Trx targets encompass, ubiquitination-related enzymes, Fe S cluster assembly proteins, phospholipases, exonucleases, and chitin synthases. Moreover, components of multiprotein complexes were co purified, reflecting both direct Trx interactions and indirect co association. Overall, this study provides a global map of potential redox regulated proteins and Trx targets in N. crassa, laying the ground for future investigations into redox signaling in filamentous fungi.