In vitro plasticity between ureteric epithelial and distal nephron identity and maturity is controlled by extracellular signals.
In vitro plasticity between ureteric epithelial and distal nephron identity and maturity is controlled by extracellular signals.
Kairath, P.; Er, P. X.; Wilson, S. B.; Ghobrial, I. T.; Vanslambrouck, J. M.; Chen, Y.-H.; Pruett-Miller, S. M.; Jain, S.; Little, M. H.
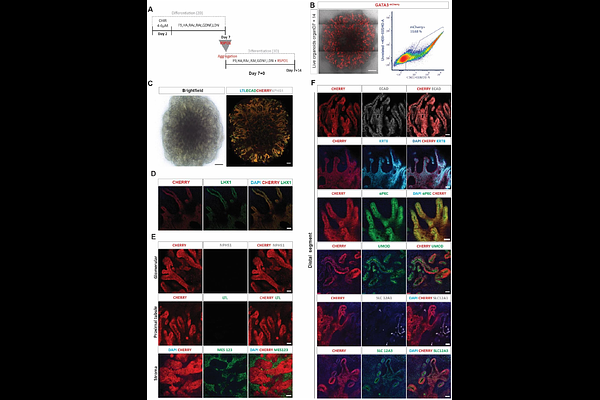
AbstractSeveral studies have described human pluripotent stem cell (hPSC)-derived ureteric epithelium, which in the embryo arises from the nephric duct and forms the collecting ducts of the kidney. However, hPSC-derived distal nephron epithelium can also adopt a ureteric phenotype, despite this not occurring during embryogenesis. In this study, RETtdTomato and GATA3mCherry reporter lines were used to further investigate this plasticity. Induction of anterior intermediate mesoderm resulted in the spontaneous formation of an epithelial plexus with a nephric duct-like identity. Subsequent addition of RSPO1 induced patterning of distalizsed nephrons, including distal convoluted tubule and thick ascending limb of loop of Henle but lacking proximal segments or glomeruli. This epithelium showed a capacity to adopt ureteric epithelial or nephric duct-like states in ex vivo co-culture in response to external cues. The same epithelium seeded as single cells in Matrigel formed epithelial spheroids and adopted a RET+ ureteric tip identity. This in vitro continuum between nephric duct, ureteric epithelium and distal nephron illustrates the role of the microenvironment in cellular identity.