A family of E3 ligases extend K11 polyubiquitin on sites of MARUbylation
A family of E3 ligases extend K11 polyubiquitin on sites of MARUbylation
Lacoursiere, R. E.; Upadhyaya, K.; Kaur Sidhu, J.; Bejan, D. S.; Rodriguez Siordia, I.; Cohen, M. S.; Pruneda, J. N.
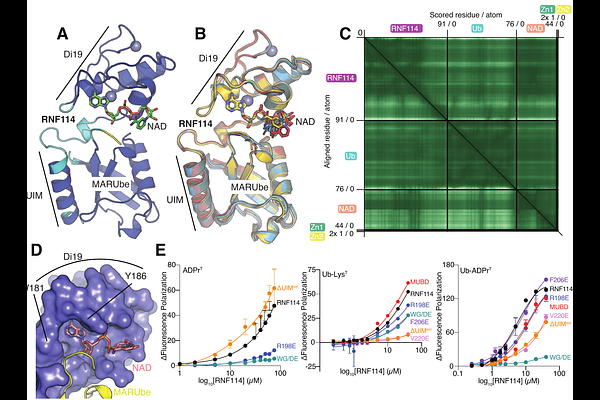
AbstractUbiquitin (Ub) cooperation with other post-translational modifications provides a tiered opportunity for protein regulation. Small modifications to Ub such as phosphorylation, acetylation, or ADP-ribosylation have varying impacts on signaling. The Deltex family of E3 ligases was previously implicated in the ubiquitylation of ADP-ribose (ADPr) and ADPr-containing macromolecules. Our previous work found ester-linked mono-ADPr ubiquitylation (MARUbylation) on PARP7 and PARP10 in cells and that this mark is extended with K11 polyUb. We previously screened for E3 ligases that interact with PARP7 through three different approaches and identified six candidates, including the Deltex family member DTX2. One of these hits, RNF114, interacts with various other PARPs, leading us to hypothesize that RNF114 binds to sites of MARUbylation and extends K11 polyUb. Here, we show that DTX2 generates the initial MARUbe on PARP7 in cells, which depends on PARP7 catalytic activity. The MARUbe on PARP7 is extended with K11 polyUb by RNF114. To investigate the mechanism of RNF114 reader/writer function, we developed a click chemistry-inspired chemoenzymatic approach to create a novel fluorescent Ub-ADPr probe for studying its interaction with RNF114. Strikingly, we found that RNF114 has a weak affinity for ADPr and Ub separately but explicitly recognizes the linkage between Ub and ADPr present in MARUbylated species. We used AlphaFold3 modeling to examine the mechanisms of Ub-ADPr recognition and K11-linked polyUb extension by RNF114. We identified a tandem Di19-UIM module in RNF114 as a MARUbe-binding domain (MUBD), thus providing a reader function that interfaces with K11-specific writer activity. Finally, we described a small family of MUBD-containing E3 ligases that demonstrate preference for Ub-ADPr, which we call MARUbe-Targeted Ligases (MUTLs).