Mycobacterial EtfD contains an unusual linear cluster and enables β-oxidation to drive proton pumping by the electron transport chain
Mycobacterial EtfD contains an unusual linear cluster and enables β-oxidation to drive proton pumping by the electron transport chain
Courbon, G. M.; Makarov, V.; Cole, S. T.; Schnappinger, D.; Ehrt, S.; Rubinstein, J. L.
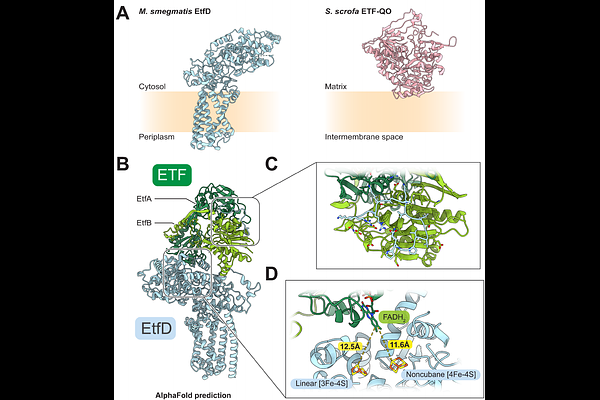
AbstractIn mycobacteria, the protein EtfD is thought to link {beta}-oxidation of fatty acids with the electron transport chain, two processes that have attracted attention as targets for therapeutics to treat tuberculosis (TB) and other mycobacterial infections. It has been proposed that targeting {beta}-oxidation could shorten treatment duration by killing non-replicating Mycobacterium tuberculosis within granulomas in the lungs. Here we show that Mycobacterium smegmatis, a fast growing and nonpathogenic model for energy metabolism in M. tuberculosis, relies on EtfD for extracting energy from {beta}-oxidation. Electron cryomicroscopy allowed structure determination of M. smegmatis EtfD, revealing an unusual linear [3Fe-4S] cluster that has not been seen in other protein structures, but which resembles the catalytic noncubane [4Fe-4S] clusters in heterodisulfide reductases. The structure suggests how EtfD transfers electrons from {beta}-oxidation to the electron transport chain. We devised an assay that couples EtfD activity to a fluorescent readout of proton pumping by the electron transport chain, which can be used to identify compounds that block mycobacteria from using {beta}-oxidation to power oxidative phosphorylation.