An optimized rabies vaccine vehicle for orotopical administration to wild vampire bats.
An optimized rabies vaccine vehicle for orotopical administration to wild vampire bats.
Knuese, C.; Cardenas-Canales, E. M.; McDevitt-Galles, T.; Ramirez-Martinez, M. M.; Limonta, D.; Powers, L. E.; Walsh, D. P.; Streicker, D. G.; Osorio, J. E.; Zamanian, M.; Rocke, T. E.
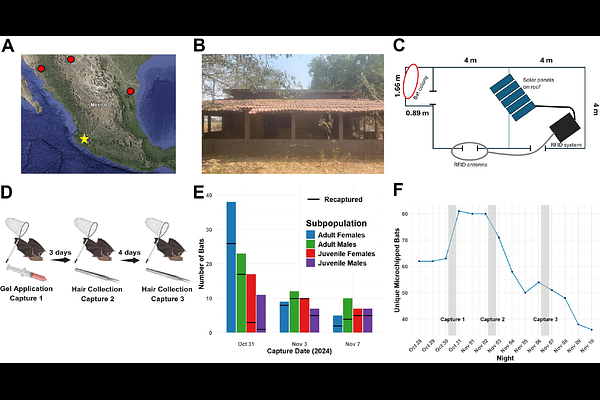
AbstractRabies vaccination of vampire bats (Desmodus rotundus) has been proposed as a superior control method to culling but has yet to be implemented. Success of rabies vaccination depends on a topical vehicle that spreads through a bat colony via allogrooming while additionally preserving vaccine immunogenicity. This work describes the in vitro and in vivo optimization of a new orotopical gel for rabies vaccine delivery to vampire bats. Autonomous transferability of our carboxymethyl cellulose (CMC) gel vaccine delivery formulation was tested in a microchipped vampire bat colony in rural Jalisco, Mexico. Intra-colony gel uptake was traced using the fluorescent biomarker rhodamine B. Importantly, application of topical treatment of ~20% of the bat colony resulted in estimated uptake by over 85% of the colony. The in vitro stability of our raccoon poxviral-vectored rabies vaccine candidate within CMC was measured at time points up to 3 months at 40 {degrees}C, 23 {degrees}C, and 4 {degrees}C. Extended storage at 4 {degrees}C and short exposure at higher temperatures of potential vampire bat environments preserved vaccine titers within CMC. Furthermore, physical properties of our CMC formulation were compared to the previously used glycerin jelly at 40 {degrees}C, 20 {degrees}C, and 0 {degrees}C using rheological tests. These tests indicate that CMC exhibits optimal properties for topical application to bats even at extreme temperatures possible during field vaccination. This study advances our rabies vaccination strategy for vampire bats by providing a topical vehicle suitable for field application that may additionally be employed for other significant bat diseases.