Loss of the alternative calcineurin variant CnAβ1 enhances brown adipocyte differentiation and drives metabolic overactivation through FoxO1 activation
Loss of the alternative calcineurin variant CnAβ1 enhances brown adipocyte differentiation and drives metabolic overactivation through FoxO1 activation
Bello-Arroyo, E.; Rubio, B.; Mora, A.; Lopez-Olaneta, M.; Gomez-Salinero, J. M.; Ramos, L.; Sanchez-Cabezudo, C. G.; Camafeita, E.; Cusso, L.; Desco, M.; Joffin, N.; Le Coq, J.; Boskovic, J.; McGurk, K. A.; Ware, J. S.; Barton, P. J.; Vazquez, J.; Scherer, P. E.; Sabio, G.; Gomez-Gaviro, M. V.; Lara-Pezzi, E.
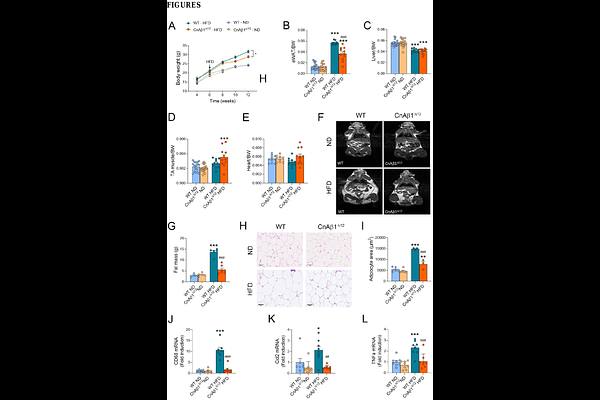
AbstractThe alternative calcineurin A variant CnA{beta}1 has a unique C-terminal domain that provides it with distinct subcellular localization and mechanism of action different from other calcineurin isoforms. Here, we used mice lacking CnA{beta}1 C-terminal domain (CnA{beta}1{Delta}i12) to show that the absence of this specific isoform strongly reprograms metabolism. CnA{beta}1{Delta}i12 mice on a high-fat diet showed reduced body weight, white adipose tissue (WAT) mass, and circulating triglycerides, together with enhanced insulin sensitivity. In brown adipose tissue (BAT), CnA{beta}1 deficiency increased mitochondrial content and upregulated fatty acid oxidation and thermogenic proteins, improving cold resistance. Conversely, under starvation, CnA{beta}1{Delta}i12 mice experienced rapid fat depletion and hypothermia. Importantly, BAT-specific FoxO1 knockout in CnA{beta}1{Delta}i12 mice reduced catabolism-related gene expression and partially reversed the metabolic phenotypes, increasing body weight and WAT mass. Our findings reveal a relevant role for CnA{beta}1 in orchestrating BAT metabolism, highlighting its potential as a therapeutic target for obesity and metabolic syndrome.