Aberrant Fibro-Adipogenic Progenitor Subpopulations Drive Volumetric Muscle Loss-Induced Fibrosis
Aberrant Fibro-Adipogenic Progenitor Subpopulations Drive Volumetric Muscle Loss-Induced Fibrosis
Anderson, S. E.; Hymel, L. A.; Zhang, H.; McKinney, J. M.; Turner, T. C.; Mohiuddin, M.; Han, W. M.; Lee, N. H.; Choi, J. J.; Jeong, G.; Greenwood, E.; Chatterjee, P.; Lee, S.; Gibson, G.; Wood, L. B.; Botchwey, E. A.; Jang, Y. C.; Willett, N. J.
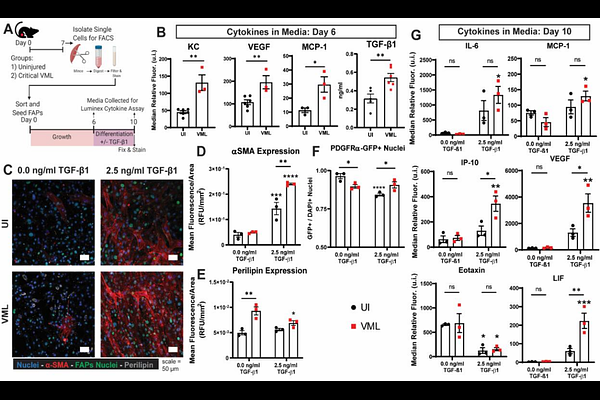
AbstractVolumetric muscle loss (VML) injuries result in chronic fibrosis, inflammation, and persistent functional deficits. Fibro-adipogenic progenitor (FAP) cells are a heterogeneous, muscle-resident stromal cell population that play a crucial role in muscle regeneration, but also contribute to fibrosis in muscle disease. The role of FAPs in VML is not well established and may be critical target to ensure functional muscle regeneration after VML. We utilized a VML model in the mouse quadriceps to study the location, secretome, surface marker distribution, gene expression, and single-cell transcriptional profile of FAPs after VML. After VML, a subpopulation of FAPs highly expressed {beta}1-integrin and were elevated in the post-VML muscle tissue; these FAPs had increased fibrotic gene expression and increased myofibroblast differentiation potential. Transforming growth factor-{beta}1 (TGF-{beta}1) and tissue inhibitor of matrix metalloproteinase 1 (TIMP1) were identified as secreted proteins from VML derived FAPs that produced both pro-fibrotic and anti-myogenic signaling. These data establish an aberrant FAP sub-population that are elevated in VML injury and provides novel targets for future scarless muscle regeneration in VML.