Correlating membrane-protein dynamics with function: Integrating bioinformatics, molecular dynamics, and single-molecule FRET
Correlating membrane-protein dynamics with function: Integrating bioinformatics, molecular dynamics, and single-molecule FRET
Higinbotham, H. R.; Arbour, C. A.; Imperiali, B.
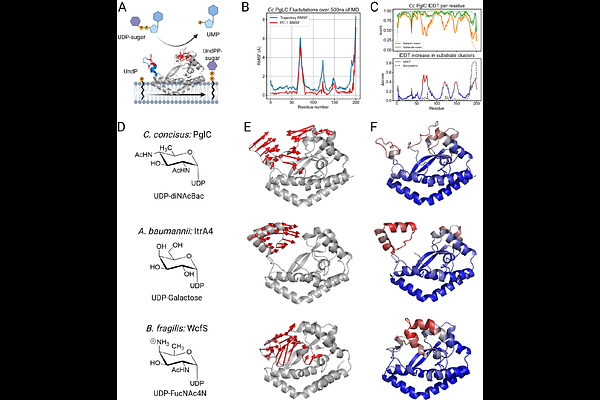
AbstractWe present a strategy that deploys structural bioinformatics, molecular simulation, and single-molecule FRET microscopy for observing the ligand-dependent conformational dynamics of integral membrane proteins in situ. We focus on representative members of the small monotopic phosphoglycosyl transferase (SmPGT) superfamily, which catalyze transfer of a phosphosugar from a soluble nucleotide-sugar donor to a membrane-embedded polyprenol phosphate acceptor in the initiating step of glycoconjugate biosynthesis in prokaryotes. Substrate-specific structural features were identified across the superfamily and correlated with ligand-dependent conformational dynamics in all-atom simulations. To experimentally validate the role of this motion in ligand binding, we developed a platform to monitor intra-molecular protein dynamics in a native-like lipid environment. The presented approach incorporates selective cysteine protein labeling and non-canonical amino acid mutagenesis with bicyclononyne-tetrazine click chemistry to assemble dual-labeled variants of PglC, the initiating enzyme of the N-linked protein glycosylation pathway from Campylobacter jejuni. The modified proteins are then solubilized into styrene maleic acid liponanoparticles (SMALPs) to maintain an in situ membrane environment. The conformational changes of PglC upon inhibitor binding are diagnostic of inhibitor potency. The single-molecule FRET-SMALP strategy can be adapted to investigate protein dynamics across the superfamily of SmPGTs with different substrate selectivity where structure prediction and molecular dynamics support significant conformational changes upon ligand binding.