Uromodulin velcro sheets and their interaction with uropathogenic E. coli
Uromodulin velcro sheets and their interaction with uropathogenic E. coli
Banjara, S.; Wang, H.; Schaeffer, C.; Stsiapanava, A.; Sandin, S.; Carroni, M.; Okumura, H.; Rampoldi, L.; Sandblad, L.; Jovine, L.
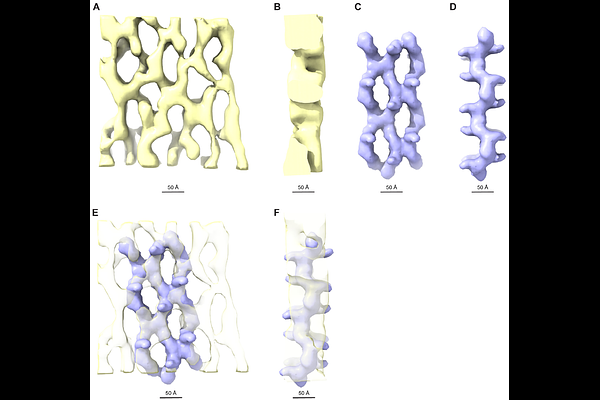
AbstractHomopolymeric uromodulin (UMOD), the most abundant protein in human urine, protects against urinary tract infections (UTI) by acting as a decoy for uropathogenic E. coli (UPEC). Using cryo-electron tomography (ET), we show that urinary UMOD filaments naturally form sheets that interact with UPEC type I pili. Sheet formation is salt-dependent, and we resolve their high-resolution structure using single-particle cryo-electron microscopy (EM). This reveals a lateral interface between polymers, whose mutation disrupts UMOD filament bundle formation in mammalian cells. Branchless egg coat protein ZPD also forms sheets, and cryo-ET of elastase-treated UMOD indicates that absence of N-terminal branches promotes the stacking of UMOD-like protein sheets into thick 3D matrices. These results rationalize early observations of salt-dependent aggregation of UMOD, explain how UMOD can assemble into extended velcro-like structures that efficiently inactivate a multitude of adhesive UPEC pili, and suggest how UMOD-like molecules can generally organize into supramolecular structures of variable thickness.