Neutralizing Activity of Cervicovaginal Secretions against Herpes Simplex Virus is Mediated by Mucosal IgG and Viral Glycoprotein E and Adversely Impacted by Vaginal Dysbiosis
Neutralizing Activity of Cervicovaginal Secretions against Herpes Simplex Virus is Mediated by Mucosal IgG and Viral Glycoprotein E and Adversely Impacted by Vaginal Dysbiosis
MAHANT MAHANT, A.; Fong, V.; Gromisch, M.; Hunte, R.; Michael, I.; Aguilan, J. T.; Murphy, K.; Keller, M. J.; Herold, B.
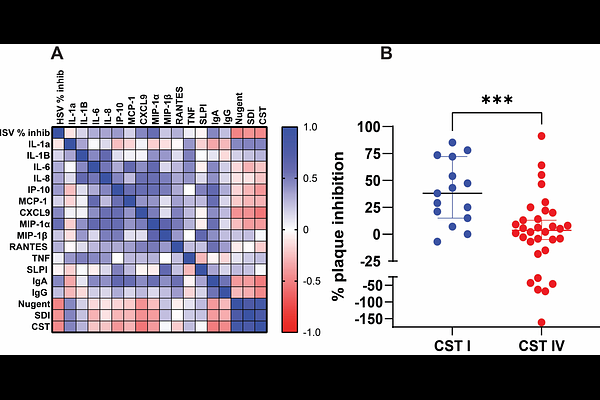
AbstractGenital herpes simplex virus (HSV) recurrences are more common in women with bacterial vaginosis (BV). Prior studies demonstrated that genital tract secretions exhibit variable neutralizing activity against HSV, independent of serostatus, but the relationship of this activity to the vaginal microbiome and underlying mechanisms have not been defined. To test the hypothesis that cervicovaginal antiviral activity is lower in women with BV, we took advantage of cervicovaginal lavage (CVL) available from two studies conducted among women with symptomatic BV and healthy controls. CVL obtained from women with BV had significantly less antiviral activity than controls (p< 0.001). Inhibitory activity correlated negatively and most strongly with Shannon diversity index (p<0.0001). The innate activity did not differ comparing HSV-seropositive versus seronegative participants and no HSV-specific antibodies were detected in CVL. Activity was enriched in the immunoglobulin fraction but was lost when IgG (but not IgA) was depleted. Increasing doses of an anti-glycoprotein E (gE) monoclonal antibody overcame the neutralizing activity, suggesting that interactions between the Fc region of IgG and gE, a viral Fc gamma receptor (FcgR), contribute. Consistent with this notion, CVL had less HSV inhibitory activity against a gE-null virus. Glycan analysis demonstrated a decrease in mature glycans in IgG from CVL with low antiviral activity and treatment of CVL with peptide N-glycanase F, which cleaves N-glycans in IgG, resulted in a loss of HSV inhibitory activity. We speculate that glycosidases elaborated by anaerobic bacteria cleave Fc glycans, resulting in decreased affinity for gE and a reduction in protective activity.