Mechanism of formin-mediated filament nucleation from profilin-actin
Mechanism of formin-mediated filament nucleation from profilin-actin
Zweifel, M. E.; Courtemanche, N.
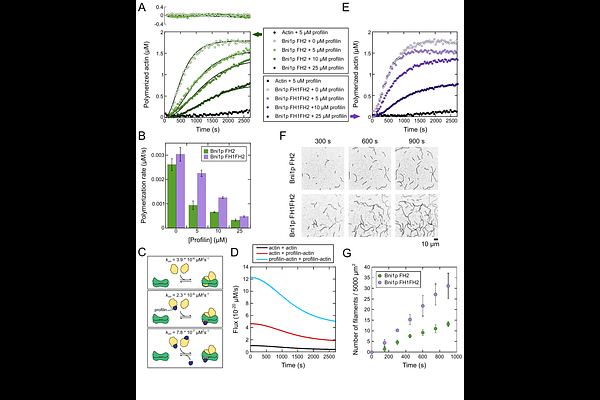
AbstractFormins direct the assembly of unbranched actin filaments through dynamic interactions with the abundant, actin-binding protein profilin. Although the process of formin-mediated filament elongation is well characterized, the mechanism by which formins nucleate actin filaments is poorly understood. In this study, we used in vitro reconstitution assays and kinetic modeling to dissect filament nucleation mediated by the S. cerevisiae formin Bni1p. We found that formins assemble filament nuclei by sequentially binding three actin monomers in a process that takes place in parallel to spontaneous actin assembly. Whereas the formin FH2 domain preferentially binds actin monomers, the flexible FH1 domain facilitates de novo filament formation by binding and transferring profilin-actin complexes to a free FH2 dimer. FH1-mediated delivery stimulates filament nucleation in a distance- and sequence-dependent manner. The architectures of FH1 domains therefore maximize the efficiency of formin-mediated polymerization through optimized engagement of each of their profilin-binding sites.