Multivalent interaction induces phase separation and formation of more toxic aggregates of α-syn in a yeast model of Parkinson's disease
Multivalent interaction induces phase separation and formation of more toxic aggregates of α-syn in a yeast model of Parkinson's disease
Chattopadhyay, K.; Jain, R.; Sharavanakkumar, S.
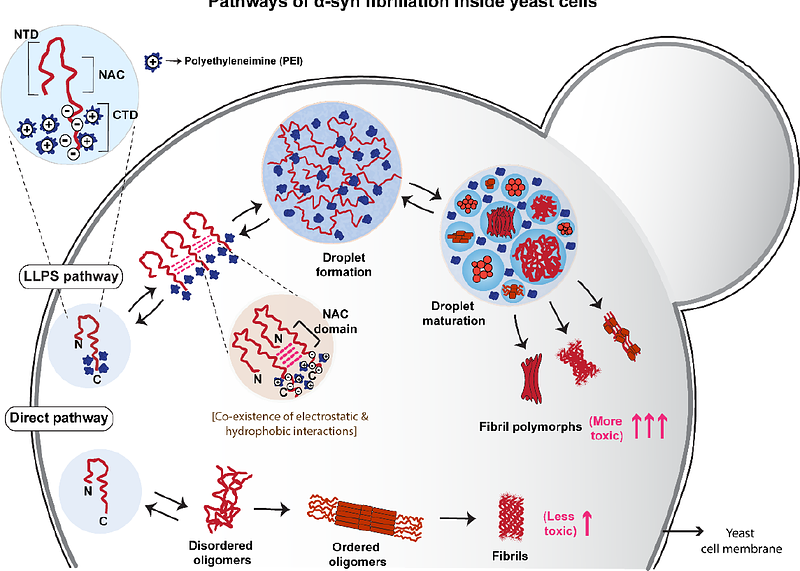
AbstractThe process of protein phase separation, particularly in the context of intrinsically disordered proteins, has been extensively studied for its implications in several neuro-degenerative diseases. Although the mechanism of protein phase separation and the involved molecular grammar have been well explored under in vitro conditions, the focus is now shifting towards developing more complex models of phase separation in order to mimic the biological systems more closely. Here, we studied the phase separation of alpha synuclein (-syn), an intrinsically disordered protein whose aggregation is implicated in the pathology of Parkinson\'s Disease, (PD) inside yeast cells (Saccharomyces cerevisiae). Using a positively charged polymer; polyethylenimine (PEI), which binds presumably at the negatively charged C-terminal domain of -syn, we find that the aggregation of -syn inside yeast can be modulated by at least two pathways: one involving phase separation and the second one without phase separation. We find further that these two pathways lead to varying fibril characteristics and toxicities. We believe that this model can be used as a quick and convenient system to screen novel and repurposed small molecules against toxic protein droplets.