The bivalent epigenetic regulator ULTRAPETALA1 promotes the Arabidopsis floral transition via recruitment of Polycomb histone methyltransferases for H3K27me3 deposition at the FLC locus
The bivalent epigenetic regulator ULTRAPETALA1 promotes the Arabidopsis floral transition via recruitment of Polycomb histone methyltransferases for H3K27me3 deposition at the FLC locus
Xing, Q.; Shemyakina, E.; Tian, J.; Strotmann, V. I.; Anwar, A.; Li, X.; Xiong, Y.; Yang, T.; Zheng, Y.; Pu, L.; Stahl, Y.; Mueller-Xing, R.; Fletcher, J. C.
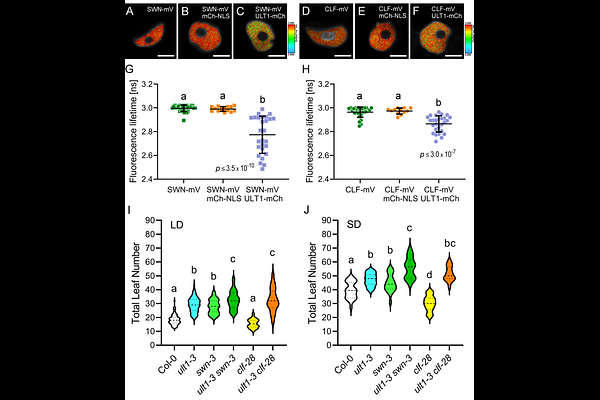
AbstractFloral induction is a major transition during the plant life cycle that contributes to reproductive fitness in annual and perennial plants. Flowering occurs in response to multiple environmental and endogenous cues. In Arabidopsis thaliana, many of these cues converge on the regulation of the FLOWERING LOCUS C (FLC) gene, which encodes a MADS domain transcription factor that functions as the main floral repressor. Here we demonstrate that the bivalent epigenetic regulator ULTRAPETALA1 (ULT1) is both necessary and sufficient to promote flowering. We identify ULT1 as a novel component of the autonomous pathway (AP) and show that it accelerates flowering by directly repressing FLC transcription. We demonstrate that ULT1 physically associates with the SWINGER (SWN) and CURLY LEAF (CLF) histone methyltransferase components of Polycomb Repressive Complex 2 (PRC2) to regulate FLC transcription by altering the accumulation of H3K27me3 marks. Our data indicate that ULT1 promotes the floral transition by interacting with SWN and CLF to recruit PRC2 to the FLC locus to deposit repressive histone methylation and reduce transcription of this key floral repressor.