WNK1 kinase activity is required for maintenance of podocyte structure
WNK1 kinase activity is required for maintenance of podocyte structure
Liu, Z.; Lee, E.; Jiang, S.; Yoon, J.; Ahmed, F.; Rahman, M. A.; Bleicher, W. P.; Suleiman, H. Y.; Bruggeman, L. A.; Miller, R. T.; Chang, A. N.
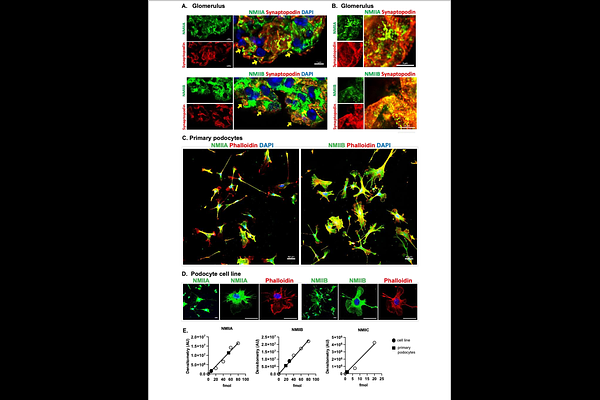
AbstractThe filtration-function of glomeruli requires slit diaphragms formed by interdigitating podocyte foot processes, which are actin-based membrane protrusions. Dysregulation of mechanisms that maintain these membrane extensions lead to foot process effacement, proteinuria, and progression to chronic kidney disease. Building on our previous work that showed WNK1 kinase activity is necessary for the maintenance of normal biomechanical properties of glomeruli and podocyte foot process architecture, we tested the hypothesis that WNK1 kinase activity affects the structure of podocyte foot processes through modulation of actomyosin activity and focal adhesion complexes. Using a WNK1 kinase specific inhibitor, we determined by immunofluorescence microscopy of nascent focal adhesions, podocyte membrane spreading/extensions, and NMII paralog localization and extent of activation calculated from quantification of phosphorylated myosin, that all were sensitive to WNK1 kinase activity. Moreover, biochemical evidence of WNK1 kinase activity-dependent signalosomes supports a role for WNK1 in the maintenance of podocyte foot processes, and sarcomere-like structures (SLSs) that are induced in models of podocyte injury. Using primary and immortalized podocyte cell lines developed from control and Col4a3-/- Alport Syndrome model mice, we measured WNK1 kinase activity-dependent improvement in properties of injured podocytes in vitro. Physiological relevance of WNK1 kinase activity-dependent structural maintenance of podocyte foot processes was confirmed by significant acute proteinuria measured in response to WNK1 inhibition in vivo. Collectively, the results provide evidence that WNK1 kinase signalosome activity that includes formation of nascent focal adhesions and regulation of NMII localization and activity at membrane protrusions and extensions, are necessary for physiological maintenance of slit diaphragms.