DNA-based delivery of incretin receptor agonists using MYO Technology leads to durable weight loss in a diet-induced obesity model
DNA-based delivery of incretin receptor agonists using MYO Technology leads to durable weight loss in a diet-induced obesity model
Sasset, L.; Cameron, A. D.; Sussman, C.; Rubinelli, L.; Maji, D.; Miller, R.; Thompson, A. T.; Campbell, D.; Walker, M. R.; Drozdz, M. M.; Liberatore, R. A.
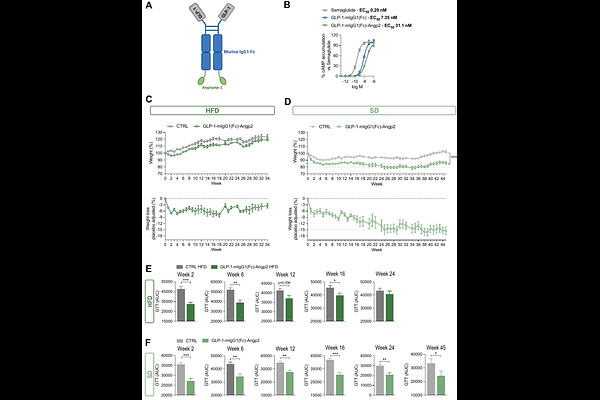
AbstractTherapeutic proteins have seen a substantial increase in clinical development and use across many disease areas. Despite their broad applicability, significant drawbacks limit access to many of these drugs, including: i) high manufacturing costs; ii) administration via time consuming infusions; iii) frequent dosing, sometimes even daily; and iv) requirements for low temperature storage. MYO Technology was developed to overcome these barriers. The MYO Technology platform consists of therapeutic-encoding plasmid DNA (pDNA), and a proprietary medical device for intramuscular injection and delivery of electrical pulses. These pulses enable the in vivo electroporation of muscle cells and uptake of injected pDNA, leading to the production, secretion, and delivery of the therapeutic protein into peripheral circulation. MYO Technology offers several advantages over standard delivery of therapeutic proteins; pDNA manufacturing is a simpler and less specialized process compared to protein manufacturing, and pDNA is very stable and lacks most cold chain requirements. Furthermore, administration using MYO Technology takes only a few minutes, and the serum level of a therapeutic protein can potentially be maintained for many months without the need for redosing. Incretin receptor agonists (IRAs) are a class of therapeutic proteins that have recently come to prominence as powerful weight and glucose control drugs, and are used for the treatment of type 2 diabetes (T2D) and obesity. Semaglutide and tirzepatide, currently the most widely used within this class, are both potent molecules, but have a short half-life, requiring weekly administration by subcutaneous injections. Moreover, since their clinical benefits rapidly disappear upon treatment cessation, T2D and obese patients may have a life-long dependency on IRAs, and the requirement for weekly injections can negatively affect the quality of life and the adherence to therapy, as well as create a significant financial burden. Therefore, increasing the interval between injections has become one of the major goals in the field. Here, we present our preclinical studies on the delivery of IRAs with MYO Technology. Animal proof of concept studies demonstrate that MYO Technology-delivered IRAs are functional, and efficacious in promoting long lasting weight and glucose control in mouse models of diet induced obesity. Moreover, engineering the IRAs to facilitate blood brain barrier penetration further enhances treatment efficacy, with benefits persisting well beyond six months following a single administration. Together, these findings highlight MYO Technology potential to transform care for patients with T2D and obesity by enabling long-lasting therapeutic effects with minimal dosing, ultimately improving quality of life and treatment adherence.