Lenacapavir-induced Lattice Hyperstabilization is Central to HIV-1 Capsid Failure at the Nuclear Pore Complex and in the Cytoplasm
Lenacapavir-induced Lattice Hyperstabilization is Central to HIV-1 Capsid Failure at the Nuclear Pore Complex and in the Cytoplasm
Hudait, A.; Burdick, R. C.; Bare, E. K.; Pathak, V. K.; Voth, G. A.
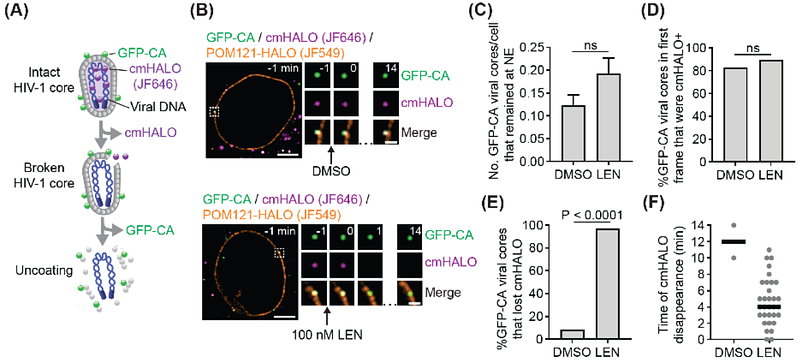
AbstractLenacapavir (LEN) is the first HIV-1 capsid inhibitor approved for clinical use.It inhibits multiple steps of the viral life cycle; however, the molecular details of the effect of LEN on capsid structure and the mechanistic steps of the inhibition are not understood. Recent studies show that intact cone-shaped capsids and capsids with LEN-induced breaks can dock at nuclear pore complexes (NPC), but only intact capsids enter the nucleus. In this work, we combined large-scale coarse-grained molecular dynamics simulations and live-cell imaging to investigate the stepwise mechanism of docking of LEN-treated capsids into the NPC. Capsids bound to substoichiometric concentrations of LEN can reach the NPC central channel. As the capsid advances to the nuclear end, lattice defects are formed at the pentamer-hexamer interface -primarily at the narrower end -leading to pentamer dissociation. Dissociation of pentamers is detrimental to capsid integrity, leading to both rupture of the narrow end and destabilization of the hexamer-hexamer interface. Structural analysis of LEN-capsid complexes in our simulations demonstrate heterogeneous hyperstabilization and loss of the essential pliability of the capsid protein lattice. Live-cell imaging of HIV-1 cores labeled with two different fluorescent markers showed that LEN-treated ruptured capsids were docked at the NPC but were not imported into the nucleus. We conclude that LEN contributes to the loss of capsid elasticity and integrity, inhibiting HIV-1 nuclear entry and replication.