Alternatively-Spliced CAMKK2 isoforms drive differential metabolic stress response and the regulation of ferroptosis
Alternatively-Spliced CAMKK2 isoforms drive differential metabolic stress response and the regulation of ferroptosis
Cheng, S.; Kim, H.; Fahmi, N. A.; Park, M.; Canny, M.; Kukreja, R.; Beckermann, E. J.; Latham, M.; Zhang, W.; Yong, J.
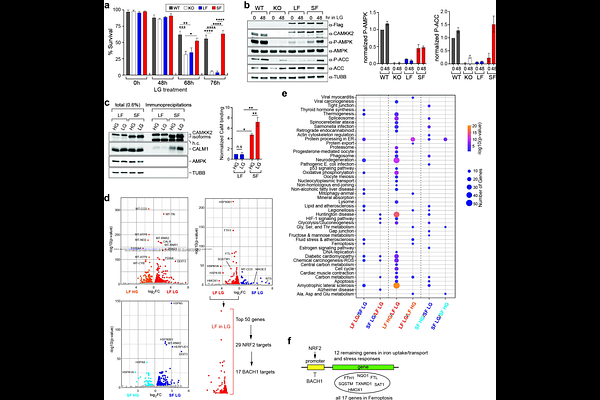
AbstractCalcium/Calmodulin-dependent protein kinase kinase 2 (CAMKK2) is a multifunctional kinase that regulates metabolic processes by phosphorylating downstream targets. CAMKK2 is expressed as several highly similar protein isoforms, although the specific functions of these individual isoforms remain largely unexplored. These isoforms have been shown to display tissue-specific expression patterns, suggesting unique roles in distinct cellular contexts. In this study, we investigated the biochemical and functional relevance of CAMKK2 isoforms, LF and SF, in the context of glucose metabolism. Co-immunoprecipitation experiments revealed that the LF isoform preferentially binds the adaptor protein 14-3-3, while the SF isoform interacts with Calmodulin. Furthermore, the interaction between SF and Calmodulin was enhanced upon glucose starvation, whereas this interaction was not observed for LF. To assess their functional significance, we generated doxycycline-inducible, isoform-specific HeLa cell lines. Under low glucose conditions, cells expressing the LF isoform failed to activate AMPK, while cells expressing the SF isoform exhibited robust increase in AMPK phosphorylation. Moreover, LF-expressing cells accumulated higher levels of reactive oxygen species, upregulated ferroptosis-related genes via BACH1/NRF2, and displayed increased cell death compared to SF-expressing cells. Collectively, these findings demonstrate that CAMKK2 isoforms exhibit differential selectivity for protein partners and mediate distinct downstream signaling pathways, impacting metabolism in cancer.