Vascular endothelial growth factor receptors 1 and 3 mediate placental trophoblast leptin production in preeclampsia, inducing vascular dysfunction
Vascular endothelial growth factor receptors 1 and 3 mediate placental trophoblast leptin production in preeclampsia, inducing vascular dysfunction
Elgazzaz, M.; Ogbi, S.; Moronge, D.; Mellott, E.; Cooper, G.; Backer, K.; Hitchings, J.; Zarate, L. V.; Kavuri, S.; Lee, T. J.; Ilatovskaya, D.; Woodham, P. C.; Maher, J.; Annex, B. H.; Faulkner, J. L.
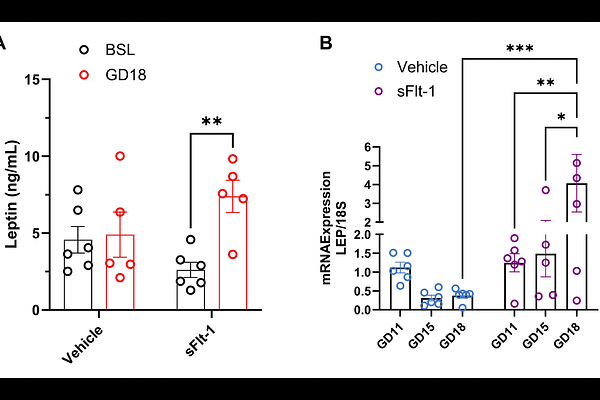
AbstractHeightened soluble FMS-like tyrosine kinase-1 (sFlt-1) levels is a hallmark of preeclampsia patients and induces a state of angiogenic imbalance by sequestering free vascular endothelial growth factor (VEGF) and placental growth factor (PlGF). The receptors for VEGF and PlGF, membrane-bound VEGFR, are expressed in placental trophoblast cells, but their functions are largely unknown. Placenta production of leptin significantly increases in preeclampsia, and we recently showed leptin induces placental and vascular endothelial dysfunction in pregnancy. We hypothesized that there is a mechanistic link in which inappropriately high sFlt-1 in preeclampsia leads to an increase in trophoblast leptin production. We treated human placental explants and trophoblast cells with sFlt-1 and show an increase in leptin peptide production, which is ablated by coadministration with either VEGF or placental growth factor (PLGF). We further demonstrate that VEGFR1 and 3, not R2, expressions are predominant in human trophoblasts and that reducing activation of these receptors mediates trophoblast leptin production. In pregnant mice, we show that sFlt-1 infusion induces vascular endothelial dysfunction in association with significantly elevated plasma leptin levels. In pregnant sFlt-1-infused mice treatment with leptin receptor antagonist significantly ablated vascular endothelial dysfunction. Collectively, these data indicate that angiogenic imbalance in preeclampsia impacts placental trophoblast endocrine function by suppressing VEGFR1 and 3 activation, resulting in leptin overproduction. Furthermore, sFlt-1 induces vascular endothelial dysfunction in mice dependent on leptin receptor activation.