Extracellular stiffness regulates cell fate determination and drives the emergence of evolutionary novelty in teleost heart
Extracellular stiffness regulates cell fate determination and drives the emergence of evolutionary novelty in teleost heart
Matsuki, S.; Inoue, Y.; Watanabe, R.; Mitsui, T.; Moriyama, Y.
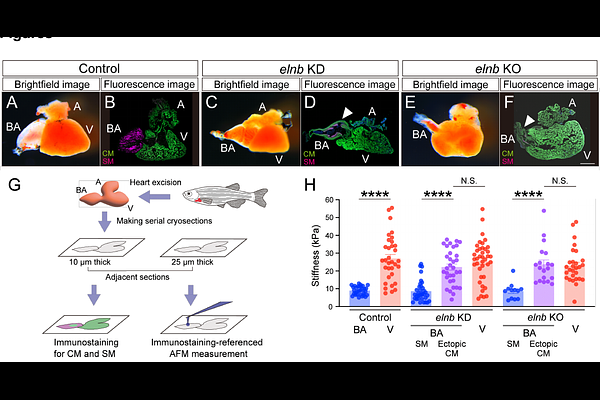
AbstractThe diversification of life is driven by the acquisition of new phenotypic traits, known as evolutionary novelties. While some genetic mechanisms underlying such traits have been identified, the role of physical properties of cellular and extracellular components remains largely unexplored. Here, we show that the evolution and development of the bulbus arteriosus (BA) - a teleost-specific, smooth muscle-rich heart outflow tract - is regulated by the mechanical properties of its extracellular environment. Specifically, we demonstrate that the teleost-specific extracellular matrix gene elastin b confers the uniquely low stiffness of the BA. In contrast to the BA, the homologous organ in non-teleost actinopterygians, the conus arteriosus (CA), and the ventricle are predominantly composed of cardiomyocytes, and exhibit higher extracellular stiffness. Loss of elastin b function by knockdown or knockout leads to ectopic cardiomyocyte formation in the BA, accompanied by increased extracellular stiffness comparable to that of the ventricle. Furthermore, artificial stiffening of the BA extracellular environment is sufficient to induce ectopic cardiomyocyte differentiation. Taken together, these findings demonstrate that extracellular stiffness governs cell fate determination and highlight its role in the emergence of evolutionary novelties in the teleost heart.