Of condensates and coats - reciprocal regulation of clathrin assembly and the growth of protein networks
Of condensates and coats - reciprocal regulation of clathrin assembly and the growth of protein networks
Malady, B.; Papagiannoula, A.; Kamatar, A.; Sarkar, S.; LeBrun, G. T.; Wang, L.; Hayden, C. C.; Lafer, E. M.; Owen, D. J.; Milles, S.; Stachowiak, J.
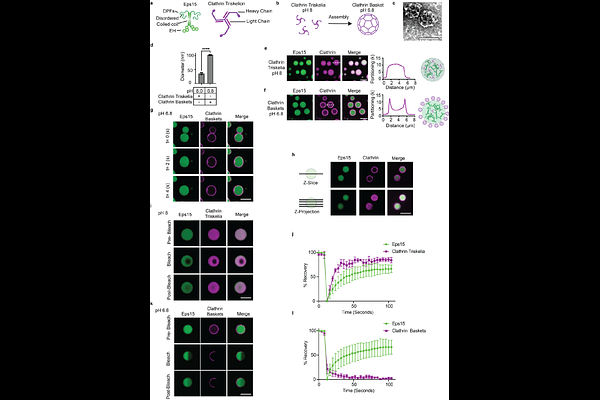
AbstractClathrin-mediated endocytosis is essential for membrane traffic, impacting a diverse range of cellular processes including cell signaling homeostasis, cell adhesion, and receptor recycling. During endocytosis, invagination of the plasma membrane is coordinated by a network of proteins that recruit and assemble the clathrin coat. Recent work demonstrated that clathrin accessory proteins which arrive early at endocytic sites, such as Eps15 and Fcho2, form phase-separated condensates that recruit downstream machinery, promoting assembly and maturation of clathrin-coated vesicles. However, the mechanisms by which protein condensates regulate - and are regulated by - clathrin assembly remain unclear. Using in vitro reconstitution and nuclear magnetic resonance spectroscopy, we demonstrate that protein condensates provide a platform for recruitment and assembly of clathrin triskelia. This condensate driven assembly is enhanced in the presence of the accessory protein, AP2, which is readily incorporated within condensates. In turn, clathrin assembly restricted the growth of condensates, exhibiting surfactant-like behavior that stabilized protein-protein interactions while imposing the preferred curvature of the clathrin lattice. This mutual regulation promotes efficient assembly of clathrin-coated vesicles while preventing uncontrolled expansion of protein condensates. More broadly, reciprocal regulation of protein condensates and clathrin coats may provide a framework for understanding how intrinsically disordered and structured protein assemblies can work together to build complex cellular architectures.