EPIGENETIC REPROGRAMMING OF CELL IDENTITY IN THE RAT PRIMARY NEURON-GLIA CULTURES INVOLVES HISTONE SEROTONYLATION
EPIGENETIC REPROGRAMMING OF CELL IDENTITY IN THE RAT PRIMARY NEURON-GLIA CULTURES INVOLVES HISTONE SEROTONYLATION
Borodinova, A. A.; Leontovich, Y. A.; Beletskiy, A. P.; Revishchin, A. V.; Pavlova, G. V.; Balaban, P. M.
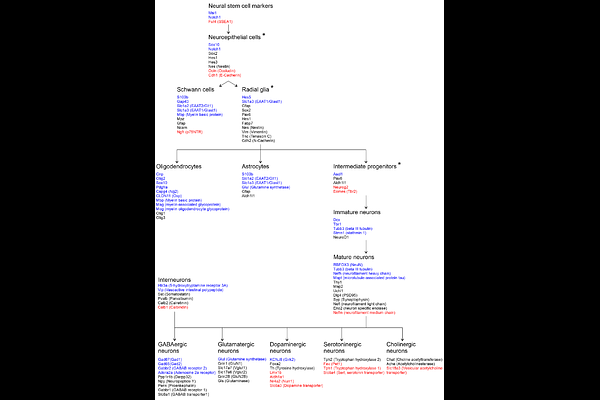
AbstractEpigenetic rearrangements can create a favorable environment for the intrinsic plasticity of brain cells, leading to cellular reprogramming into virtually any cell type through the induction of cell-specific transcriptional programs. In this study, we assessed how chromatin remodeling induced by broad-spectrum HDAC inhibitors affects cellular differentiation trajectories in rat primary neuron-glia cultures using a combination of transcriptomics, qPCR and cytochemistry. We described epigenetic regulation of transcriptional programs controlled by master transcription factors and neurotrophins in the context of neuronal and glial differentiation and evaluated the expression of representative cell-specific markers. The results obtained suggest that HDAC inhibitors reduce proliferative potential of cultured cells and induce transcriptomic changes associated with cell differentiation and specialization. Particularly, we revealed a significant upregulation of genes typically expressed in neuromodulatory neurons, and downregulation of genes expressed in glia and inhibitory neurons. Transcriptional changes were accompanied by continuous elevation of histone serotonylation levels in both neurons and glia. We assume that early appearance of the enhanced histone serotonylation marks, and the persistence of these changes over many hours in distinct brain cells may indicate that chromatin remodeling induced by histone serotonylation contributes to the maintainance of a new transcriptional programs associated with cellular reprogramming.