Constitutive Androstane Receptor induces Ribonucleotide Reductase-M2 expression and maintains hepatocyte ploidy in mice
Constitutive Androstane Receptor induces Ribonucleotide Reductase-M2 expression and maintains hepatocyte ploidy in mice
Asokaumar, A.; Mathur, B.; Chou, A.; Cronologia, B.; Alencastro, F.; Wheeler, L.; Mathews, C. K.; Moore, D. D.; Duncan, A. W.; Anakk, S.
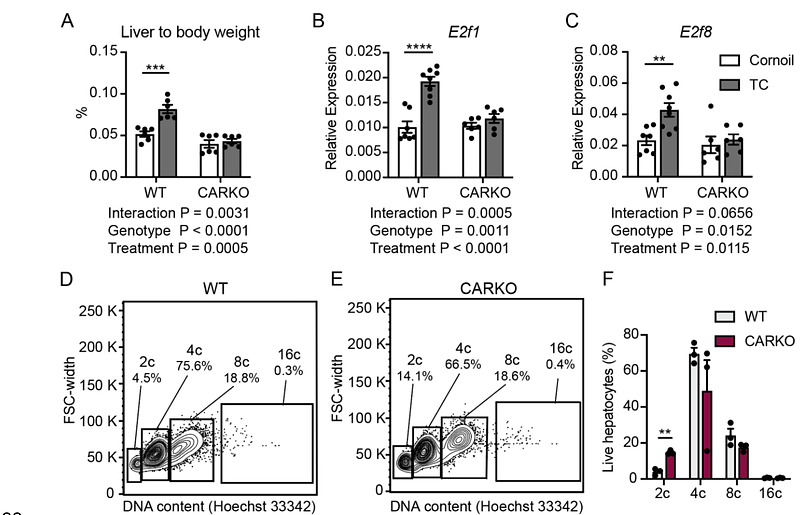
AbstractThe nuclear receptor Constitutive Androstane Receptor (CAR/NR1i3) is known for regulating various liver functions, including detoxification, nutrient metabolism, and hepatocyte proliferation. While CAR activation has been previously linked to higher ploidy, the underlying mechanisms are not fully known. Here, we uncover a basal role for CAR in maintaining hepatocyte ploidy, such that CAR deletion increases the number of diploid (2c) hepatocytes with a concomitant reduction in tetraploid (4c) hepatocytes. We demonstrate that CAR controls the de novo dNTP synthesis by directly transactivating the Ribonucleotide reductase-M2 (RRM2) gene, which encodes the rate-limiting catalytic subunit of the enzyme, ribonucleotide reductase. Further, we find that the ligand-dependent CAR activation is sufficient to induce several genes involved in the de novo dNTP synthesis pathways, resulting in higher hepatic dATP and dTTP levels within the liver. Importantly, overexpressing RRM2 levels in CAR knockouts led to increased DNA synthesis and tetraploid (4c) hepatocytes compared to the control mice. Taken together, these findings reveal that the CAR-mediated RRM2 regulation contributes towards DNA synthesis and thereby maintains hepatocyte ploidy.