Structural Basis of Herpesvirus Helicase-Primase Inhibition by Pritelivir and Amenamevir
Structural Basis of Herpesvirus Helicase-Primase Inhibition by Pritelivir and Amenamevir
Baranovskiy, A. G.; He, Q.; Suwa, Y.; Morstadt, L. M.; Babayeva, N. D.; Lim, C. J.; Tahirov, T. H.
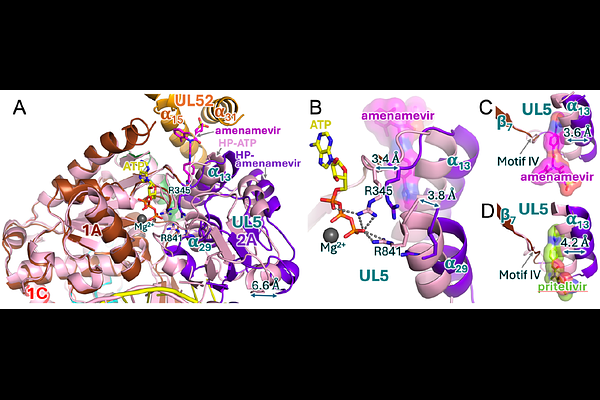
AbstractWidespread herpesvirus infections are associated with various diseases. DNA replication of human herpes simplex virus type 1 (HSV-1) requires a helicase-primase (HP) complex of three core proteins: UL5, UL52, and UL8. This complex unwinds viral DNA and synthesizes primers for DNA replication, making it an attractive antiviral target. Although HP inhibitors pritelivir and amenamevir were identified through screening, their binding mechanisms remain unclear. Here, we report cryo-EM structures of HSV-1 HP bound to a forked DNA template alone, and in complex with pritelivir or amenamevir. The structures reveal a bilobed architecture highlighting helicase-primase coordinated action at the replication fork and providing a structural basis for HP inhibition by illustrating precisely how pritelivir and amenamevir block helicase activity. Data lays a solid foundation for the development of improved antiviral therapies.