Structure of the energy converting methyltransferase (Mtr) of Methanosarcina mazei in complex with an oxygen-stress responsive small protein
Structure of the energy converting methyltransferase (Mtr) of Methanosarcina mazei in complex with an oxygen-stress responsive small protein
Reif-Trauttmansdorff, T.; Herdering, E.; Bohn, S.; Pascoa, T.; Kahnt, J.; Zimmer, E.; Kumar, A.; Schmitz, R. A.; Schuller, J. M.
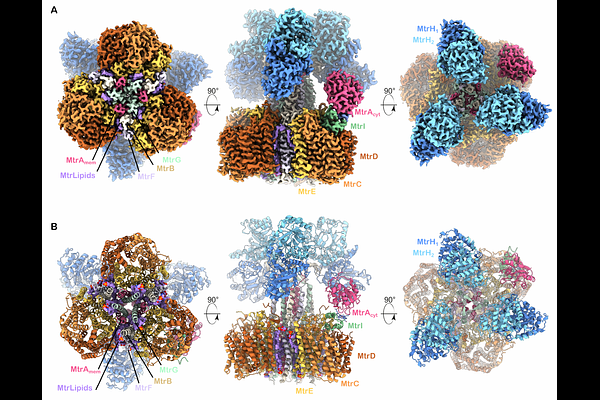
AbstractMethanogenic archaea contribute 1-2 Gt of methane annually, impacting both global carbon cycling and climate. Central to their energy metabolism is a membrane [ndash]bound, sodium-translocating methyltransferase complex: the Na+ tetrahydromethanopterin:CoM-S [ndash]methyltransferase (Mtr complex), which catalyzes the methyl transfer between two methanogen specific cofactors. This exergonic methyl transfer step is coupled with a vectorial sodium ion transport from the cytoplasm to the cell exterior and is the only energy conserving step in hydrogenotrophic methanogenesis. Here, we present a 2.1 [A] single-particle cryo-EM structure of the full Mtr complex from Methanosarcina mazei. Our structural model encompasses the entire complex, reveals the arrangement of archaea phospholipids, the architecture of the sodium ion binding site, and the structure and interactions of all catalytic subunits. Most strikingly, we discover and characterize MtrI, a previously unannotated small open reading frame (small ORF), encoded protein (<100 aa) conserved across the order of Methanosarcinales. MtrI binds to the cytoplasmic domain of MtrA in response to oxygen exposure, suggesting a role in oxygen stress response and protection. By binding on top of the sodium channel and anchoring to the cobalamid cofactor in MtrA\'s cytoplasmic domain, MtrI might prevent sodium leakage and inhibit MtrA-CoM turnover. These findings offer new insights into methanogen energy conservation and uncover a potential adaptive response to oxygen exposure, expanding our understanding of methanogen survival strategies under oxidative stress.