Sufficient levels of BECLIN1 are required for intestinal epithelial cell homeostasis and protection against unwanted intestinal inflammation
Sufficient levels of BECLIN1 are required for intestinal epithelial cell homeostasis and protection against unwanted intestinal inflammation
Juliani, J.; Tran, S.; Harris, T. J.; Ellis, S. L.; Al-Ani, A. H.; Patel, K. M.; Young, S. N.; Evangelista, M.; Baloyan, D.; Reehorst, C. M.; Nightingale, R.; Jenkins, L. J.; De Cruz, P.; Duszyc, K.; Kile, B. T.; Yap, A. S.; Mariadason, J. M.; Christensen, B.; Samson, A. L.; Murphy, J. M.; Fairlie, W. D.; Lee, E. F.
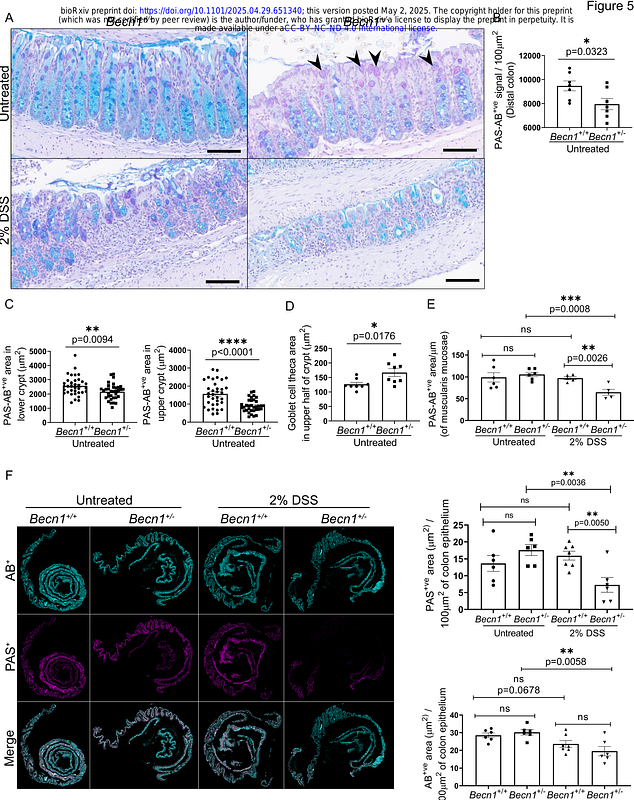
AbstractThe prototypical autophagy regulator BECLIN1, orchestrates both autophagic and endocytic trafficking, and its homozygous deletion in the intestinal epithelium leads to intestinal disruption bearing similarities to inflammatory bowel disease (IBD). However, complete loss of BECLIN1 is rare in human disease. To model the effects of a partial reduction in BECLIN1, we examined mice with a monoallelic deletion of Becn1 in the intestinal epithelium, which decreased BECLIN1 protein levels by approximately 50%. Unlike the fatal phenotype following homozygous Becn1 deletion, the heterozygous mice were grossly normal, though they presented with significantly shorter small intestines. Gastrointestinal organoids derived from the mice also displayed disrupted endocytic trafficking and altered cytoskeletal dynamics resulting in mislocalisation of junctional proteins such as E-CADHERIN. In the mice, these changes were associated with impaired goblet cell function and maturation accompanied by abnormal mucin production, composition and secretion, resulting in compromised mucus barrier integrity. The mice also exhibited heightened susceptibility to dextran sulfate sodium (DSS)-induced colitis, emphasising the critical role of BECLIN1 in mediating protection against unregulated inflammation. Investigation of BECLIN1 levels in biopsies from patients with inflammatory bowel disease (IBD) revealed changes in its expression across distinct states of inflammation with reduced levels observed in highly inflamed regions in some patients, consistent with the gastrointestinal defects seen in mice with reduced BECLIN1. Hence, this study provides crucial insights into the multifaceted importance of BECLIN1 sufficiency in the intestinal epithelium to prevent pathophysiology. This highlights the potential of BECLIN1 as a biomarker and therapeutic target for IBD management.