The Rationalization of Carbon Monoxide and Hemoglobin Association
The Rationalization of Carbon Monoxide and Hemoglobin Association
Lee, C.; Chen, N.
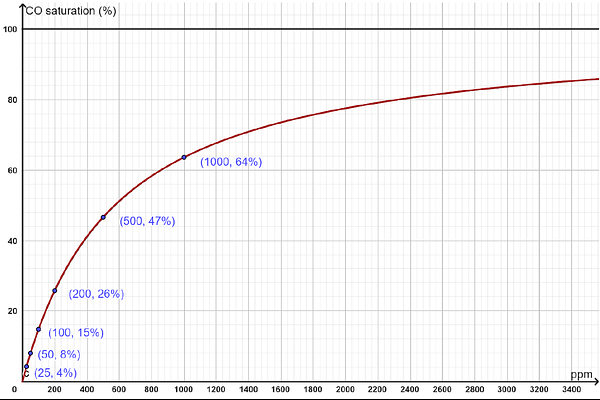
AbstractCarbon monoxide is a colorless, odorless, and poisonous gas, responsible for approximately 100,000 emergency room visits and over 420 deaths in the U.S. each year. Both carbon monoxide and oxygen bind to the ferrous ions in hemoglobin, but carbon monoxide has a significantly higher affinity. Extensive research has been conducted on the interaction between carbon monoxide and hemoglobin. However, a straightforward and practically applicable equation describing the relationship between carbon monoxide saturation and pressure is not found in the existing literature. In this paper, we establish an equation and confirm that the plot of CO saturation against CO pressure follows a hyperbolic shape, characterized by a continuous decrease in slope. In contrast, the oxygen-hemoglobin association curve is sigmoidal. These distinct curve shapes have different physiological implications. Our equation enables the determination of one variable, either saturation or pressure, if the other is known. Further analysis reveals the distribution of all five species of carboxyhemoglobin, showing that the triply bound form is abundant, a notable contrast to the distribution of oxyhemoglobin species. Additionally, our equation confirms that carbon monoxide\'s affinity for hemoglobin is approximately 230 times higher than that of oxygen. Lastly, we propose a new general equation that may generate all carbon monoxide-hemoglobin association curves under various oxygen pressures.