Targeting of CIP4-Calcineurin Signalosomes Improves Cardiac Structure and Function After Myocardial Infarction
Targeting of CIP4-Calcineurin Signalosomes Improves Cardiac Structure and Function After Myocardial Infarction
Samuelsson, A.-M.; Bayer, A. L.; Li, J.; Li, Y.; Lewis, D.; Turcotte, M.; Dodge-Kafka, K.; Alcaide, P.; Kapiloff, M. S.
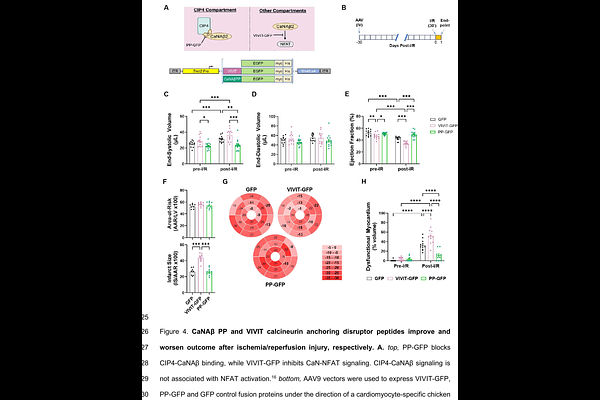
AbstractBackground: Calcineurin in a pleiotropic signaling enzyme that promotes pathological cardiac remodeling but also cardioprotection in ischemia-reperfusion injury. In addition, calcineurin inhibitors are immunosuppressants. This pleiotropy has precluded the use of calcineurin inhibitors as treatments for heart failure. Cdc42-interacting protein 4 (CIP4/TRIP10) is an endosomal scaffold protein that organizes a calcium and calcineurin A{beta}2 (CaNA{beta}2) signaling compartment activated by G-protein coupled receptors independently of contractile calcium. CIP4 binds CaNA{beta}2 via the CaNA{beta}-specific N-terminal polyproline (PP) domain. We previously showed that targeting of CIP4-CaNA{beta}2 signalosomes inhibited pathological cardiac hypertrophy and the development of heart failure induced by chronic pressure overload in mice. It is unknown whether CIP4-CaNA{beta}2 signalosomes contribute to cardioprotection and/or cardiac remodeling in ischemic heart disease. Methods: CIP4 conditional knock-out (CKO) mice were studied by echocardiography with strain analysis and histology following ischemia-reperfusion (I/R) injury and permanent left coronary artery (LCA) ligation to induce myocardial infarction. Wildtype C57BL/6NJ mice were transduced with adeno-associated virus (AAV) engineered for cardiomyocyte-specific expression of either a CaNA{beta}2 shRNA to inhibit CaNA{beta}2 expression, a VIVIT peptide to inhibit CaN-NFAT signaling, or a CaNA{beta}2 PP peptide to block CIP4-CaNA{beta}2 binding. AAV-transduced mice were studied by I/R injury. Additional mice were subjected to permanent LCA ligation and subsequently treated with AAV to test the effects of CaN inhibition in chronic ischemic cardiomyopathy. The effects of CaNA{beta}2 PP-GFP expression on primary T-cell activation were studied in vitro. Results: CIP4 CKO mice and mice expressing the PP anchoring disruptor peptide exhibited preserved cardiac function after I/R injury and decreased infarct size and preserved cardiac function 8 weeks after myocardial infarction by permanent LCA ligation. In contrast, cardiomyocyte-specific depletion of CaNA{beta}2 and VIVIT peptide expression worsened outcome after I/R injury and in chronic ischemic cardiomyopathy. In addition, in contrast to cardiomyocytes, PP-mediated CaNA{beta} anchoring inhibition had no effect on T-cell activation and cytokine expression in vitro. Conclusions: CIP4-CaNA{beta}2 signalosomes promote adverse cardiac remodeling and are not cardioprotective. Proof-of-concept is provided for the treatment of ischemic cardiomyopathy by a PP anchoring disruptor gene therapy. Targeting these complexes may be beneficial in cardiovascular diseases, including ischemic cardiomyopathy and acute myocardial infarction.