NUSAP1 regulates mitotic processes via KIF2C interaction and AURKA phosphorylation in primary microcephaly
NUSAP1 regulates mitotic processes via KIF2C interaction and AURKA phosphorylation in primary microcephaly
Ren, D.; Li, K.; Liu, C.; Bi, Y.; Liu, L.; Ji, L.; Yang, K.; Luo, Y.; Luo, L.; Yan, Y.; Li, Y.; Yang, F.; Wang, H.; He, G.; Mao, X.
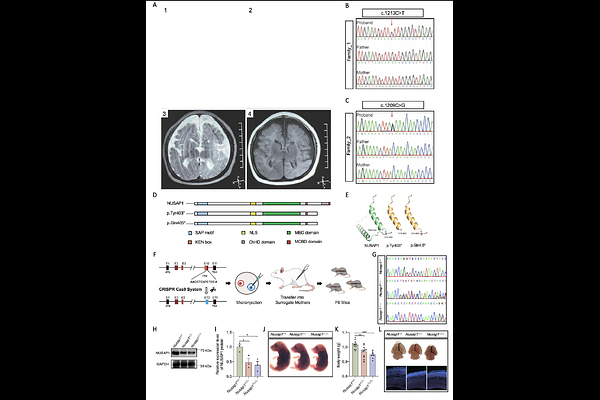
AbstractPrimary microcephaly (PM) is a neurodevelopmental disorder characterized by a significantly smaller head than normal. Despite the identification of several genes associated with PM subtypes, the etiology remains unclear in a significant proportion of patients. Here, we reported two de novo nonsense mutations in the NUSAP1 gene identified in two independent PM families: c.1209 C>G (p.Tyr403*) and c.1213C>T (p.Gln405*). Nusap1-edited mouse models, designed to mimic Human NUSAP1 mutations, replicated the microcephaly phenotype, characterized by a small brain and thinner cerebral cortex. We demonstrated that Nusap1 was specifically expressed in neural stem/progenitor cells (NSPCs), and the number of NSPCs was significantly reduced in Nusap1-edited mice. In vitro experiments revealed that NUSAP1 mutations disrupted mitotic metaphase chromosome alignment, increased polyploidy, induced cell cycle arrest, and promoted apoptosis. Co-IP confirmed direct interactions between NUSAP1 and KIF2C, and showed that truncating mutations impaired their binding. Furthermore, NUSAP1 rescued the cell cycle arrest and increased apoptosis caused by KIF2C overexpression in NUSAP1 KO HEK293T cells, whereas the mutant truncated protein failed to exert such a rescue effect. We also uncovered an interaction between NUSAP1 and Aurora kinase A (AURKA). Intriguingly, subsequent experiments revealed that AURKA-mediated phosphorylation of NUSAP1 modulates its binding affinity to KIF2C. In conclusion, our findings established NUSAP1 as a novel pathogenic gene for PM. We proposed a model in which AURKA-mediated phosphorylation of NUSAP1 regulates its interaction with KIF2C, balancing spindle microtubule stability and depolymerization to ensure proper sister chromatid segregation. Disruptions in this pathway impair NSPCs mitosis, thereby affecting neocortical development.