Discovery of late intermediates in methylenomycin biosynthesis active against drug-resistant Gram-positive bacterial pathogens
Discovery of late intermediates in methylenomycin biosynthesis active against drug-resistant Gram-positive bacterial pathogens
Corre, C.; Idowu, G.; Song, L.; Whitehead, M.; Alkhalaf, L.; Challis, G.
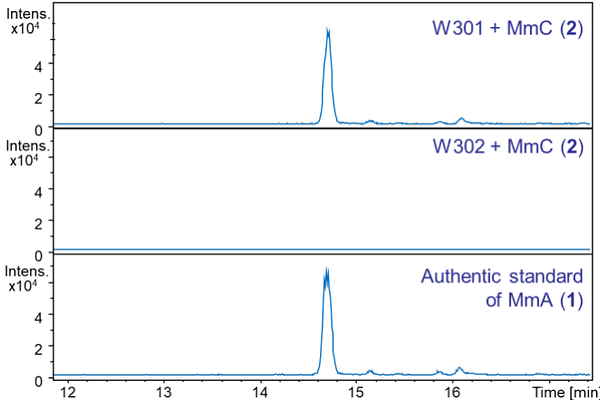
AbstractThe methylenomycins are highly functionalized cyclopentanone antibiotics produced by Streptomyces coelicolor A3(2). A biosynthetic pathway to the methylenomycins has been proposed based on sequence analysis of the proteins encoded by the methylenomycin biosynthetic gene cluster and incorporation of labelled precursors. However, the roles played by putative biosynthetic enzymes remain experimentally uninvestigated. Here, the biosynthetic functions of enzymes encoded by mmyD, mmyO, mmyF and mmyE were investigated by creating in-frame deletions in each gene and investigating the effect on methylenomycin produc-tion. No methylenomycin-related metabolites were produced by the mmyD mutant, consistent with the proposed role of MmyD in an early biosynthetic step. The production of methylenomycin A, but not methylenomycin C, was abolished in the mmyF and mmyO mutants, consistent with the corresponding enzymes catalyzing epoxidation of methylenomycin C, as previously proposed. Expression of mmyF and mmyO in a S. coelicolor M145 derivative engineered to express mmr, which confers methylenomycin resistance, enabled the resulting strain to convert methylenomycin C to methylenomycin A, confirming this hypothesis. A novel metabolite (pre-methylenomycin C), which readily cyclizes to form the corresponding butanolide (pre-methylenomycin C lactone), accumulated in the mmyE mutant, indicating the corresponding enzyme is involved in introducing the exomethylene group into methylenomycin C. Remarkably, both pre-methylenomycin C and its lactone precursor were one to two orders of magnitude more active against various Gram-positive bacteria, including antibiotic-resistant Staphylococcus aureus and Enterococcus faecium isolates, than methylenomycins A and C, providing a promising starting point for the development of novel antibiotics to combat antimicrobial resistance.