Anthrax toxin receptor 2 is the Receptor for Clostridium perfringens NetF: Structural Insights into Toxin Binding and Pore Formation
Anthrax toxin receptor 2 is the Receptor for Clostridium perfringens NetF: Structural Insights into Toxin Binding and Pore Formation
Chang, W.; Cattalani, F.; Iacovache, I.; Naguleswaran, A.; Farhoosh, F.; Franzen, J.; Abrami, L.; van der Goot, F. G.; Posthaus, H.; Zuber, B.
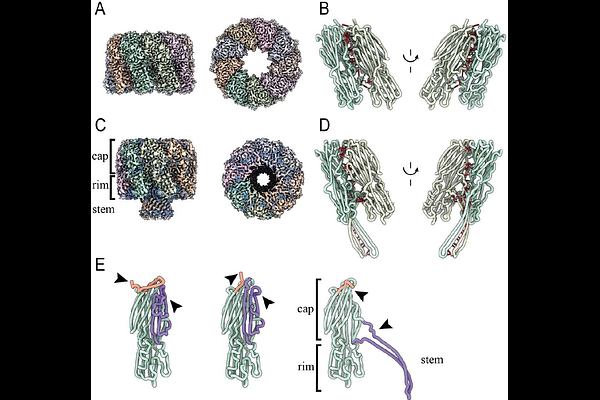
AbstractHemolysin {beta}-pore-forming toxins ({beta}PFTs) are key virulence factors of Clostridium perfringens, associated with severe diseases in humans and animals. Yet, the mechanisms by which Clostridium {beta}PFTs recognize and engage specific target cells remain poorly understood. Here, we identify the cellular receptor for C. perfringens necrotizing enteritis toxin F (NetF), a recently discovered toxin implicated in severe enteritis in dogs and foals. We show that NetF binds to the same receptor as anthrax toxin, namely ANTXR2. Using cryo-electron microscopy, we determined the structure of the oligomeric NetF pre-pore as well as the transmembrane pore, both alone and in complex with the extracellular domain of ANTXR2. Unlike anthrax toxin, which binds to the apical MIDAS motif of ANTXR2 - as does the natural ANTXR2 ligand collagen type VI - NetF engages the receptor laterally, spanning both the von Willebrand A and the Ig-like domains. This interaction positions the toxin near the membrane, facilitating contact with membrane lipids and promoting transmembrane pore formation. Our findings uncover key principles of hemolysin {beta}PFT-receptor recognition and advance our understanding of how pathogenic bacteria use these toxins to breach host defenses.