Membrane association prevents premature degradation and mitigates inefficient biogenesis of suboptimal membrane proteins
Membrane association prevents premature degradation and mitigates inefficient biogenesis of suboptimal membrane proteins
Diwo, C.; Alenquer, M.; Adrain, C.; Amorim, M. J.
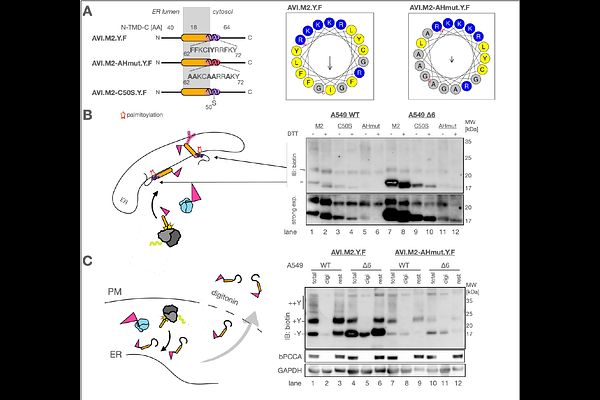
AbstractAccurate membrane protein biogenesis is essential for cellular function, yet many proteins contain suboptimal targeting or insertion signals. Influenza A virus (IAV) faces similar constraints during infection but may have evolved strategies to enhance the biogenesis of its own membrane proteins. One such protein, the viroporin M2, contains functionally essential hydrophilic residues within its transmembrane domain, which should hinder efficient membrane insertion. We hypothesize that IAV has adapted to overcome these sequence-based limitations and ensure robust M2 biogenesis. Using a biotin pulse-labeling system in intact cells, we uncover the dynamics of M2 targeting and ER insertion. Attenuating the insertion rate by ablating a key insertion factor, the ER membrane protein complex (EMC), leads to M2 accumulating in the cytosol, but remaining partially insertion competent. We find that cytosolic stability of non-inserted M2 is crucial for efficient biogenesis under these conditions and identify amphipathic helix-mediated membrane association as the molecular mechanism that counteracts proteasomal degradation. We propose membrane association as a novel buffering mechanism that regulates membrane protein biogenesis by stabilizing pre-insertion intermediates.