A myosin hypertrophic cardiomyopathy mutation disrupts the super-relaxed state and boosts contractility by enhanced actin attachment
A myosin hypertrophic cardiomyopathy mutation disrupts the super-relaxed state and boosts contractility by enhanced actin attachment
Cail, R. C.; Barua, B.; Baez-Cruz, F. A.; Winkelmann, D. A.; Goldman, Y. E.; Ostap, E. M.
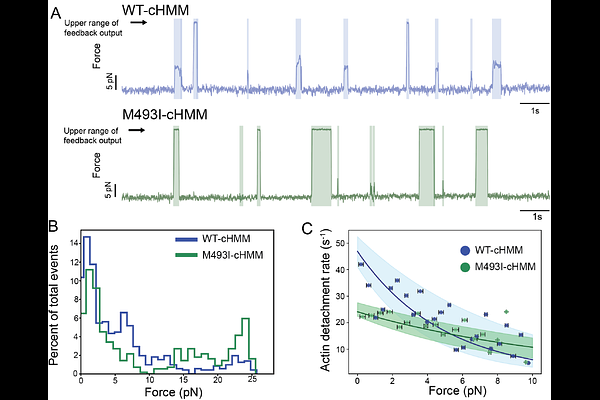
AbstractHypertrophic cardiomyopathy (HCM) is a leading cause of cardiac failure among individuals under 35. Many genetic mutations that cause HCM enhance ventricular systolic function, suggesting that these HCM mutations are hypercontractile. Among the most common causes of HCM are mutations in the gene MYH7, which encodes for beta-cardiac myosin, the principal human ventricular myosin. Previous work has demonstrated that, for purified myosins, some MYH7 mutations are gain-of-function while others cause reduced function, so how they lead to enhanced contractility is not clear. Here, we have characterized the mechanics and kinetics of the severe HCM-causing mutation M493I. Motility assays demonstrate a 70% reduction of actin filament gliding velocities on M493I-coated surfaces relative to WT. This mutation slows ADP release from actomyosin.ADP 5-fold without affecting phosphate release or ATP binding. Yet it enhances steady-state ATPase Vmax 2-fold. Through single-molecule mechanical studies, we find that M493I myosin has a normal working stroke of 5 nm but a significantly prolonged actin attachment duration. Under isometric feedback, M493I myosins produce high, sustained force, with an actin detachment rate that is less sensitive to force than that of WT myosin. We also report direct measurement of the equilibrium state of the super-relaxed to disordered relaxed (SRX-DRX) regulatory transition and show its disruption in M493I, with a concomitant enhancement to actin attachment kinetics. Together, these data demonstrate that enhanced myosin binding from inhibition of myosin\'s off state, combined with slow ADP release and enhanced force production, underlie the enhanced function and etiology of this HCM mutation.