Nonmuscle myosin 2 turnover in cells is synergistically controlled by the tail and the motor domain
Nonmuscle myosin 2 turnover in cells is synergistically controlled by the tail and the motor domain
Chougule, A.; Svitkina, T.
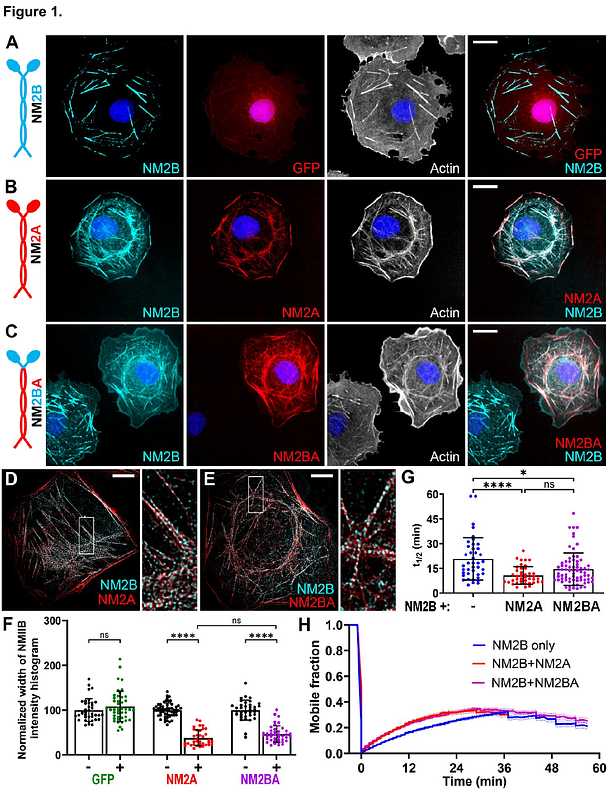
AbstractMyosin 2, an actin-dependent motor, is universally responsible for cell contractility due to its ability to form bipolar filaments. Fast turnover of nonmuscle myosin 2 (NM2) filaments is necessary to keep up with cell motility and shape changes. The turnover mechanisms are not fully understood and differ for two main mammalian paralogs -- NM2A and NM2B -- whereas paralog copolymerization adds complexity to this process by enabling the intrinsically fast NM2A to dynamize the intrinsically slow NM2B. Here, we show that the nonhelical tail, the C-terminal phosphorylation sites, and surprisingly, the motor domain of the NM2A heavy chain synergistically accelerate the turnover of NM2B in trans and cell motility, suggesting that these three mechanisms collectively control NM2A own dynamics. Conversely, the phosphomimetic NM2B tail facilitates only local turnover of endogenous wild type NM2B but not its global redistribution unless the NM2A motor is combined with the phosphomimetic NM2B tail. Collectively, we reveal the cooperation between the motor activity and the NM2 tail-targeting turnover mechanisms in regulating the NM2 filament turnover in trans and cell motility.