Identification and characterization of a small-molecule inhibitor of the Pseudomonas aeruginosa SOS response
Identification and characterization of a small-molecule inhibitor of the Pseudomonas aeruginosa SOS response
Vascon, F.; Fongaro, B.; Mickevicius, V.; Pasquato, A.; Grybaite, B.; Petraitis, V.; Polverino de Laureto, P.; Tondi, D.; Kavaliauskas, P.; Cendron, L.
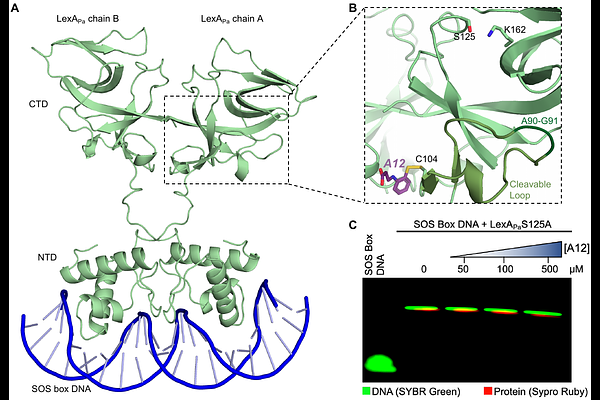
AbstractThe SOS response is among the most conserved pathways that promote the acquisition of antibiotic resistance in bacteria. This study aimed to identify and characterize small-molecule inhibitors of the SOS response system in the opportunistic pathogen Pseudomonas aeruginosa. A library of 318 drug-like compounds was screened for inhibition of RecA-induced LexA autoproteolysis, a key step in SOS activation. One hit compound, 3-(2-sulfanylanilino)propanoic acid, showed dose-dependent inhibition with an IC50 in the mid-micromolar range. Differential scanning fluorimetry and isothermal titration calorimetry analysis revealed that A12 binds to both RecA and LexA with low micromolar affinity. Mass spectrometry analysis demonstrated that A12 covalently modifies RecA likely via condensation, while it forms a disulfide bond with Cys104 of LexA. Inhibition was diminished under reducing conditions, confirming disulfide formation is crucial for A12 activity. Importantly, A12 did not impair LexA's ability to bind SOS box DNA sequences, which is needed to keep the SOS genes repressed. While A12's potency requires optimization, it represents a promising scaffold for developing anti-SOS compounds targeting P. aeruginosa.